Crystal Structure of Human Cd1e Reveals a Groove Suited for Lipid-Exchange Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fine Tuning by Human Cd1e of Lipid-Specific Immune Responses
Fine tuning by human CD1e of lipid-specific immune responses Federica Facciottia, Marco Cavallaria, Catherine Angénieuxb, Luis F. Garcia-Allesc, François Signorino-Gelob, Lena Angmana, Martine Gilleronc, Jacques Prandic, Germain Puzoc, Luigi Panzad, Chengfeng Xiae, Peng George Wangf, Paolo Dellabonag, Giulia Casoratig, Steven A. Porcellih, Henri de la Salleb, Lucia Moria,i,1, and Gennaro De Liberoa,1 aExperimental Immunology, Department of Biomedicine, University Hospital Basel, 4031 Basel, Switzerland; bInstitut National de la Santé et de la Recherche Médicale, Unité Mixte de Recherche S725, Biology of Human Dendritic Cells, Université de Strasbourg and Etablissement Français du Sang-Alsace, 67065 Strasbourg, France; cCentre National de la Recherche Scientifique, Institut de Pharmacologie et de Biologie Structurale, 31077 Toulouse, France; dDepartment of Chemistry, Food, Pharmaceuticals, and Pharmacology, Università del Piemonte Orientale, 28100 Novara, Italy; eState Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan 650204, China; fDepartment of Biochemistry and Chemistry, The Ohio State University, Columbus, OH 43210; gExperimental Immunology Unit, Division of Immunology, Transplantation, and Infectious Diseases, Dipartimento di Biotecnologie (DIBIT), San Raffaele Scientific Institute, 20132 Milano, Italy; hAlbert Einstein College of Medicine, Bronx, NY 10461; and iSingapore Immunology Network, Agency for Science Technology and Research, Biopolis, Singapore 138648 Edited by Peter Cresswell, Yale University School of Medicine, New Haven, CT, and approved July 20, 2011 (received for review June 5, 2011) CD1e is a member of the CD1 family that participates in lipid an- molecules traffic to early recycling endosomes and, to a lesser tigen presentation without interacting with the T-cell receptor. -

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

CD200, Human Recombinant Recombinant Human CD200, : 408-493-1800 | Fax: 408-493-1801 408-493-1801 Fax: | 408-493-1800
BioVision 1/14 For research use only CD200, human recombinant CATALOG #: 7309-50 50 µg ALTERNATE NAMES: CD200 molecule, MOX1, MOX2, MRC, OX-2 SOURCE: E. coli PURITY: > 90% by SDS-PAGE MOL. WEIGHT: 24.8 kDa (225 aa, 31-232 aa + His tag), confirmed by MALDI-TOF. ENDOTOXIN LEVEL: < 1.0 EU per 1 µg of protein FORM: Liquid FORMULATION: 1 mg/ml in 20 mM Tris-HCl buffer (pH 8.0) containing 0.4M Urea. CD200, human recombinant STORAGE CONDITIONS: Can be stored at 4°C short term (1-2 weeks). For long term storage, aliquot and store at -20°C or - 70°C. Avoid repeated freezing and thawing RELATED PRODUCTS: cycles. • CD1E, human recombinant (Cat. No. 7308-100) • CD226, human recombinant (Cat. No. 7310-100) DESCRIPTION : CD200 is a type-1 membrane glycoprotein, which contains two • CD274, mouse recombinant (Cat. No. 7311-100) immunoglobulin domains, and thus belongs to the immunoglobulin superfamily. Studies of • CD300C, human recombinant (Cat. No. 7312-100) the related genes in mouse and rat suggest that this gene may regulate myeloid cell • CD3G, human recombinant (Cat. No. 7313-100) activity and delivers an inhibitory signal for the macrophage lineage in diverse tissues. • CD46, human recombinant (Cat. No. 7314-100) Multiple alternatively spliced transcript variants that encode different isoforms have been • CD5, human recombinant (Cat. No. 7315-100) • CD7, human recombinant (Cat. No. 7316-100) found for this gene. Recombinant human CD200 protein, fused to His-tag at N-terminus, • CD74, human recombinant (Cat. No. 7317-100) was expressed in E.coli. • CD79B, human recombinant (Cat. -

Dux4r, Znf384r and PAX5-P80R Mutated B-Cell Precursor Acute
Acute Lymphoblastic Leukemia SUPPLEMENTARY APPENDIX DUX4r , ZNF384r and PAX5 -P80R mutated B-cell precursor acute lymphoblastic leukemia frequently undergo monocytic switch Michaela Novakova, 1,2,3* Marketa Zaliova, 1,2,3* Karel Fiser, 1,2* Barbora Vakrmanova, 1,2 Lucie Slamova, 1,2,3 Alena Musilova, 1,2 Monika Brüggemann, 4 Matthias Ritgen, 4 Eva Fronkova, 1,2,3 Tomas Kalina, 1,2,3 Jan Stary, 2,3 Lucie Winkowska, 1,2 Peter Svec, 5 Alexandra Kolenova, 5 Jan Stuchly, 1,2 Jan Zuna, 1,2,3 Jan Trka, 1,2,3 Ondrej Hrusak 1,2,3# and Ester Mejstrikova 1,2,3# 1CLIP - Childhood Leukemia Investigation Praguerague, Czech Republic; 2Department of Paediatric Hematology and Oncology, Second Faculty of Medicine, Charles University, Prague, Czech Republic; 3University Hospital Motol, Prague, Czech Republic; 4Department of In - ternal Medicine II, University Hospital Schleswig-Holstein, Kiel, Germany and 5Comenius University, National Institute of Children’s Dis - eases, Bratislava, Slovakia *MN, MZ and KF contributed equally as co-first authors. #OH and EM contributed equally as co-senior authors. ©2021 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol. 2020.250423 Received: February 18, 2020. Accepted: June 25, 2020. Pre-published: July 9, 2020. Correspondence: ESTER MEJSTRIKOVA - [email protected] Table S1. S1a. List of antibodies used for diagnostic immunophenotyping. Antibody Fluorochrome Clone Catalogue number Manufacturer CD2 PE 39C1.5 A07744 Beckman Coulter CD3 FITC UCHT1 1F-202-T100 Exbio CD4 PE-Cy7 -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Flow Reagents Single Color Antibodies CD Chart
CD CHART CD N° Alternative Name CD N° Alternative Name CD N° Alternative Name Beckman Coulter Clone Beckman Coulter Clone Beckman Coulter Clone T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells T Cells B Cells Granulocytes NK Cells Macrophages/Monocytes Platelets Erythrocytes Stem Cells Dendritic Cells Endothelial Cells Epithelial Cells CD1a T6, R4, HTA1 Act p n n p n n S l CD99 MIC2 gene product, E2 p p p CD223 LAG-3 (Lymphocyte activation gene 3) Act n Act p n CD1b R1 Act p n n p n n S CD99R restricted CD99 p p CD224 GGT (γ-glutamyl transferase) p p p p p p CD1c R7, M241 Act S n n p n n S l CD100 SEMA4D (semaphorin 4D) p Low p p p n n CD225 Leu13, interferon induced transmembrane protein 1 (IFITM1). p p p p p CD1d R3 Act S n n Low n n S Intest CD101 V7, P126 Act n p n p n n p CD226 DNAM-1, PTA-1 Act n Act Act Act n p n CD1e R2 n n n n S CD102 ICAM-2 (intercellular adhesion molecule-2) p p n p Folli p CD227 MUC1, mucin 1, episialin, PUM, PEM, EMA, DF3, H23 Act p CD2 T11; Tp50; sheep red blood cell (SRBC) receptor; LFA-2 p S n p n n l CD103 HML-1 (human mucosal lymphocytes antigen 1), integrin aE chain S n n n n n n n l CD228 Melanotransferrin (MT), p97 p p CD3 T3, CD3 complex p n n n n n n n n n l CD104 integrin b4 chain; TSP-1180 n n n n n n n p p CD229 Ly9, T-lymphocyte surface antigen p p n p n -

Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association Between BMI and Adult-Onset Non- Atopic
Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non- Atopic Asthma Ayoung Jeong 1,2, Medea Imboden 1,2, Akram Ghantous 3, Alexei Novoloaca 3, Anne-Elie Carsin 4,5,6, Manolis Kogevinas 4,5,6, Christian Schindler 1,2, Gianfranco Lovison 7, Zdenko Herceg 3, Cyrille Cuenin 3, Roel Vermeulen 8, Deborah Jarvis 9, André F. S. Amaral 9, Florian Kronenberg 10, Paolo Vineis 11,12 and Nicole Probst-Hensch 1,2,* 1 Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; [email protected] (A.J.); [email protected] (M.I.); [email protected] (C.S.) 2 Department of Public Health, University of Basel, 4001 Basel, Switzerland 3 International Agency for Research on Cancer, 69372 Lyon, France; [email protected] (A.G.); [email protected] (A.N.); [email protected] (Z.H.); [email protected] (C.C.) 4 ISGlobal, Barcelona Institute for Global Health, 08003 Barcelona, Spain; [email protected] (A.-E.C.); [email protected] (M.K.) 5 Universitat Pompeu Fabra (UPF), 08002 Barcelona, Spain 6 CIBER Epidemiología y Salud Pública (CIBERESP), 08005 Barcelona, Spain 7 Department of Economics, Business and Statistics, University of Palermo, 90128 Palermo, Italy; [email protected] 8 Environmental Epidemiology Division, Utrecht University, Institute for Risk Assessment Sciences, 3584CM Utrecht, Netherlands; [email protected] 9 Population Health and Occupational Disease, National Heart and Lung Institute, Imperial College, SW3 6LR London, UK; [email protected] (D.J.); [email protected] (A.F.S.A.) 10 Division of Genetic Epidemiology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] 11 MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, W2 1PG London, UK; [email protected] 12 Italian Institute for Genomic Medicine (IIGM), 10126 Turin, Italy * Correspondence: [email protected]; Tel.: +41-61-284-8378 Int. -

Supplemental Information For
Supplemental Information for: Gene Expression Profiling of Pediatric Acute Myelogenous Leukemia Mary E. Ross, Rami Mahfouz, Mihaela Onciu, Hsi-Che Liu, Xiaodong Zhou, Guangchun Song, Sheila A. Shurtleff, Stanley Pounds, Cheng Cheng, Jing Ma, Raul C. Ribeiro, Jeffrey E. Rubnitz, Kevin Girtman, W. Kent Williams, Susana C. Raimondi, Der-Cherng Liang, Lee-Yung Shih, Ching-Hon Pui & James R. Downing Table of Contents Section I. Patient Datasets Table S1. Diagnostic AML characteristics Table S2. Cytogenetics Summary Table S3. Adult diagnostic AML characteristics Table S4. Additional T-ALL characteristics Section II. Methods Table S5. Summary of filtered probe sets Table S6. MLL-PTD primers Additional Statistical Methods Section III. Genetic Subtype Discriminating Genes Figure S1. Unsupervised Heirarchical clustering Figure S2. Heirarchical clustering with class discriminating genes Table S7. Top 100 probe sets selected by SAM for t(8;21)[AML1-ETO] Table S8. Top 100 probe sets selected by SAM for t(15;17) [PML-RARα] Table S9. Top 63 probe sets selected by SAM for inv(16) [CBFβ-MYH11] Table S10. Top 100 probe sets selected by SAM for MLL chimeric fusion genes Table S11. Top 100 probe sets selected by SAM for FAB-M7 Table S12. Top 100 probe sets selected by SAM for CBF leukemias (whole dataset) Section IV. MLL in combined ALL and AML dataset Table S13. Top 100 probe sets selected by SAM for MLL chimeric fusions irrespective of blast lineage (whole dataset) Table S14. Class discriminating genes for cases with an MLL chimeric fusion gene that show uniform high expression, irrespective of blast lineage Section V. -
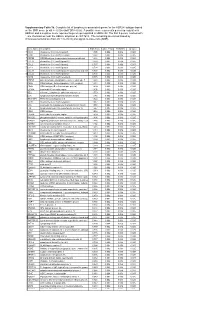
* Supplementary Table 3B. Complete List of Lymphocytic-Associated Genes
Supplementary Table 3b. Complete list of lymphocytic-associated genes for the HER2+I subtype based on the SNR score (p-val <= 0.005 and FDR<=0.05). A positive score represents genes up-regulated in HER2+I and a negative score represents genes up-regulated in HER2+NI. The first 9 genes, marked with *, are chemokines near the HER2+ amplicon at chr17q12. The remaining are sorted based by chromosomal location (from chr 1 to chr X) and signal-to-noise ratio (SNR). Gene Name Description SNR Score Feature P value FDR(BH) Q Value * CCL5 chemokine (C-C motif) ligand 5 1.395 0.002 0.036 0.025 * CCR7 chemokine (C-C motif) receptor 7 1.362 0.002 0.036 0.025 * CD79B CD79B antigen (immunoglobulin-associated beta) 1.248 0.002 0.036 0.025 * CCL13 chemokine (C-C motif) ligand 13 1.003 0.002 0.036 0.025 * CCL2 chemokine (C-C motif) ligand 2 0.737 0.004 0.056 0.037 * CCL8 chemokine (C-C motif) ligand 8 0.724 0.002 0.036 0.025 * CCL18 chemokine (C-C motif) ligand 18 (pulmonary and activ 0.703 0.002 0.036 0.025 * CCL23 chemokine (C-C motif) ligand 23 0.701 0.002 0.036 0.025 * CCR6 chemokine (C-C motif) receptor 6 0.715 0.002 0.036 0.025 PTPN7 protein tyrosine phosphatase, non-receptor type 7 1.861 0.002 0.036 0.025 CD3Z CD3Z antigen, zeta polypeptide (TiT3 complex) 1.811 0.002 0.036 0.025 CD48 CD48 antigen (B-cell membrane protein) 1.809 0.002 0.036 0.025 IL10RA interleukin 10 receptor, alpha 1.806 0.002 0.036 0.025 SELL selectin L (lymphocyte adhesion molecule 1) 1.773 0.002 0.036 0.025 LCK lymphocyte-specific protein tyrosine kinase 1.745 0.002 0.036 0.025 -

Natural Killer Cell Lymphoma Shares Strikingly Similar Molecular Features
Leukemia (2011) 25, 348–358 & 2011 Macmillan Publishers Limited All rights reserved 0887-6924/11 www.nature.com/leu ORIGINAL ARTICLE Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic cd T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro J Iqbal1, DD Weisenburger1, A Chowdhury2, MY Tsai2, G Srivastava3, TC Greiner1, C Kucuk1, K Deffenbacher1, J Vose4, L Smith5, WY Au3, S Nakamura6, M Seto6, J Delabie7, F Berger8, F Loong3, Y-H Ko9, I Sng10, X Liu11, TP Loughran11, J Armitage4 and WC Chan1, for the International Peripheral T-cell Lymphoma Project 1Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE, USA; 2Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center, Omaha, NE, USA; 3Departments of Pathology and Medicine, University of Hong Kong, Queen Mary Hospital, Hong Kong, China; 4Division of Hematology and Oncology, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA; 5College of Public Health, University of Nebraska Medical Center, Omaha, NE, USA; 6Departments of Pathology and Cancer Genetics, Aichi Cancer Center Research Institute, Nagoya University, Nagoya, Japan; 7Department of Pathology, University of Oslo, Norwegian Radium Hospital, Oslo, Norway; 8Department of Pathology, Centre Hospitalier Lyon-Sud, Lyon, France; 9Department of Pathology, Samsung Medical Center, Sungkyunkwan University, Seoul, Korea; 10Department of Pathology, Singapore General Hospital, Singapore and 11Penn State Hershey Cancer Institute, Pennsylvania State University College of Medicine, Hershey, PA, USA Natural killer (NK) cell lymphomas/leukemias are rare neo- Introduction plasms with an aggressive clinical behavior. -

Human CD Marker Chart Reviewed by HLDA1 Bdbiosciences.Com/Cdmarkers
BD Biosciences Human CD Marker Chart Reviewed by HLDA1 bdbiosciences.com/cdmarkers 23-12399-01 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD1a R4, T6, Leu6, HTA1 b-2-Microglobulin, CD74 + + + – + – – – CD93 C1QR1,C1qRP, MXRA4, C1qR(P), Dj737e23.1, GR11 – – – – – + + – – + – CD220 Insulin receptor (INSR), IR Insulin, IGF-2 + + + + + + + + + Insulin-like growth factor 1 receptor (IGF1R), IGF-1R, type I IGF receptor (IGF-IR), CD1b R1, T6m Leu6 b-2-Microglobulin + + + – + – – – CD94 KLRD1, Kp43 HLA class I, NKG2-A, p39 + – + – – – – – – CD221 Insulin-like growth factor 1 (IGF-I), IGF-II, Insulin JTK13 + + + + + + + + + CD1c M241, R7, T6, Leu6, BDCA1 b-2-Microglobulin + + + – + – – – CD178, FASLG, APO-1, FAS, TNFRSF6, CD95L, APT1LG1, APT1, FAS1, FASTM, CD95 CD178 (Fas ligand) + + + + + – – IGF-II, TGF-b latency-associated peptide (LAP), Proliferin, Prorenin, Plasminogen, ALPS1A, TNFSF6, FASL Cation-independent mannose-6-phosphate receptor (M6P-R, CIM6PR, CIMPR, CI- CD1d R3G1, R3 b-2-Microglobulin, MHC II CD222 Leukemia -

TSLP-Induced Mechanisms and Potential Therapies for CRLF2 B-Cell Acute Lymphoblastic Leukemia Olivia L
Loma Linda University TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works Loma Linda University Electronic Theses, Dissertations & Projects 6-2015 TSLP-induced Mechanisms and Potential Therapies for CRLF2 B-cell Acute Lymphoblastic Leukemia Olivia L. Francis Follow this and additional works at: http://scholarsrepository.llu.edu/etd Part of the Anatomy Commons, Genetic Phenomena Commons, Hemic and Lymphatic Diseases Commons, and the Medical Anatomy Commons Recommended Citation Francis, Olivia L., "TSLP-induced Mechanisms and Potential Therapies for CRLF2 B-cell Acute Lymphoblastic Leukemia" (2015). Loma Linda University Electronic Theses, Dissertations & Projects. 282. http://scholarsrepository.llu.edu/etd/282 This Dissertation is brought to you for free and open access by TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. It has been accepted for inclusion in Loma Linda University Electronic Theses, Dissertations & Projects by an authorized administrator of TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. For more information, please contact [email protected]. LOMA LINDA UNIVERSITY School of Medicine in conjunction with the Faculty of Graduate Studies ____________________ TSLP-induced Mechanisms and Potential Therapies for CRLF2 B-cell Acute Lymphoblastic Leukemia by Olivia L Francis ____________________ A Dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Anatomy ____________________