2018 07 29 Bardera Et Al Shrimp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shrimp Farming in the Asia-Pacific: Environmental and Trade Issues and Regional Cooperation
Shrimp Farming in the Asia-Pacific: Environmental and Trade Issues and Regional Cooperation Recommended Citation J. Honculada Primavera, "Shrimp Farming in the Asia-Pacific: Environmental and Trade Issues and Regional Cooperation", trade and environment, September 25, 1994, https://nautilus.org/trade-an- -environment/shrimp-farming-in-the-asia-pacific-environmental-and-trade-issues-- nd-regional-cooperation-4/ J. Honculada Primavera Aquaculture Department Southeast Asian Fisheries Development Center Tigbauan, Iloilo, Philippines 5021 Tel 63-33-271009 Fax 63-33-271008 Presented at the Nautilus Institute Workshop on Trade and Environment in Asia-Pacific: Prospects for Regional Cooperation 23-25 September 1994 East-West Center, Honolulu Abstract Production of farmed shrimp has grown at the phenomenal rate of 20-30% per year in the last two decades. The leading shrimp producers are in the Asia-Pacific region while the major markets are in Japan, the U.S.A. and Europe. The dramatic failures of shrimp farms in Taiwan, Thailand, Indonesia and China within the last five years have raised concerns about the sustainability of shrimp aquaculture, in particular intensive farming. After a brief background on shrimp farming, this paper reviews its environmental impacts and recommends measures that can be undertaken on the farm, 1 country and regional levels to promote long-term sustainability of the industry. Among the environmental effects of shrimp culture are the loss of mangrove goods and services as a result of conversion, salinization of soil and water, discharge of effluents resulting in pollution of the pond system itself and receiving waters, and overuse or misuse of chemicals. Recommendations include the protection and restoration of mangrove habitats and wild shrimp stocks, management of pond effluents, regulation of chemical use and species introductions, and an integrated coastal area management approach. -

Effects of Environmental Stress on Shrimp Innate Immunity and White
Fish and Shellfish Immunology 84 (2019) 744–755 Contents lists available at ScienceDirect Fish and Shellfish Immunology journal homepage: www.elsevier.com/locate/fsi Full length article Effects of environmental stress on shrimp innate immunity and white spot syndrome virus infection T ∗ Yi-Hong Chenb,c, Jian-Guo Hea,b, a State Key Laboratory for Biocontrol, School of Life Sciences, Sun Yat-sen University, 135 Xingang Road West, Guangzhou, 510275, PR China b Key Laboratory of Marine Resources and Coastal Engineering in Guangdong Province/School of Marine Sciences, Sun Yat-sen University, 135 Xingang Road West, Guangzhou, 510275, PR China c Guangzhou Key Laboratory of Subtropical Biodiversity and Biomonitoring, Guangdong Provincial Key Laboratory for Healthy and Safe Aquaculture, College of Life Science, South China Normal University, Guangzhou 510631, PR China ARTICLE INFO ABSTRACT Keywords: The shrimp aquaculture industry is plagued by disease. Due to the lack of deep understanding of the relationship Shrimp between innate immune mechanism and environmental adaptation mechanism, it is difficult to prevent and Environmental stress control the diseases of shrimp. The shrimp innate immune system has received much recent attention, and the Innate immunity functions of the humoral immune response and the cellular immune response have been preliminarily char- Unfolded protein response acterized. The role of environmental stress in shrimp disease has also been investigated recently, attempting to White spot syndrome virus clarify the interactions among the innate immune response, the environmental stress response, and disease. Both the innate immune response and the environmental stress response have a complex relationship with shrimp diseases. Although these systems are important safeguards, allowing shrimp to adapt to adverse environments and resist infection, some pathogens, such as white spot syndrome virus, hijack these host systems. -

Machrobrachium Rosenbergii)
RESEARCH ARTICLE Biomolecular changes that occur in the antennal gland of the giant freshwater prawn (Machrobrachium rosenbergii) Utpal Bose1,2¤, Thanapong Kruangkum3,4, Tianfang Wang1, Min Zhao1, Tomer Ventura1, Shahida Akter Mitu1, Mark P. Hodson2,5, Paul N. Shaw5, Prasert Sobhon4,6, Scott F. Cummins1* 1 Genetic, Ecology and Physiology Centre, Faculty of Science, Health, Education and Engineering, University of the Sunshine Coast, Maroochydore DC, Queensland, Australia, 2 Metabolomics Australia, a1111111111 Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, a1111111111 Queensland, Australia, 3 Department of Anatomy, Faculty of Science, Mahidol University, Bangkok, a1111111111 Thailand, 4 Center of Excellence for Shrimp Molecular Biology and Biotechnology (Centex Shrimp), Faculty a1111111111 of Science, Mahidol University, Bangkok, Thailand, 5 S chool of Pharmacy, The University of Queensland, a1111111111 Queensland, Australia, 6 Faculty of Allied Health Sciences, Burapha University, Chonburi, Thailand ¤ Current address: CSIRO Agriculture and Food, Queensland, Australia * [email protected] OPEN ACCESS Abstract Citation: Bose U, Kruangkum T, Wang T, Zhao M, Ventura T, Mitu SA, et al. (2017) Biomolecular In decapod crustaceans, the antennal gland (AnG) is a major primary source of externally changes that occur in the antennal gland of the giant freshwater prawn (Machrobrachium secreted biomolecules, and some may act as pheromones that play a major role in aquatic rosenbergii). PLoS ONE 12(6): e0177064. https:// animal communication. In aquatic crustaceans, sex pheromones regulate reproductive doi.org/10.1371/journal.pone.0177064 behaviours, yet they remain largely unidentified besides the N-acetylglucosamine-1,5-lac- Editor: Gao-Feng Qiu, Shanghai Ocean University, tone (NAGL) that stimulates male to female attraction. -

Sensory Systems and Feeding Behaviour of the Giant Freshwater Prawn, Macrobrachium Rosenbergii, and the Marine Whiteleg Shrimp, Litopenaeus Vannamei
Borneo Journal of Marine Science and Aquaculture Volume: 01 | December 2017, 80 - 91 Sensory systems and feeding behaviour of the giant freshwater prawn, Macrobrachium rosenbergii, and the marine whiteleg shrimp, Litopenaeus vannamei Gunzo Kawamura1*, Teodora Uy Bagarinao2 and Annita Seok Kian Yong1 1Borneo Marine Research Institute, Universiti Malaysia Sabah, 88400 Kota Kinabalu, Sabah, Malaysia 2Aquaculture Department, Southeast Asian Fisheries Development Center, Tigbauan, Iloilo, Philippines *Corresponding author: [email protected] Abstract Information on the sensory basis of shrimp feeding provides the means for assessment of the effectiveness of food items in terms of smell, taste, size, and colour. This chapter summarizes information about the sensory basis of the feeding behaviour of the giant freshwater prawn (Macrobrachium rosenbergii) and the marine whiteleg shrimp (Litopenaeus vannamei). Existing literature on these shrimp species and other decapod crustaceans is reviewed, and unpublished experiments using the selective sensory ablation technique to determine the involvement of vision, chemoreception, and touch sense in the feeding behavior of the juveniles of M. rosenbergii and L. vannamei are also described. To determine the role of vision in feeding, the eyes of the juveniles were painted over (deprived of vision) with white manicure and their feeding response to commercial pellets was compared with those with untreated eyes. The untreated eyed juveniles detected and approached a feed pellet right away, but the specimens blinded by the coating detected a pellet only after random accidental touch with the walking legs while roaming on the aquarium bottom. Juveniles that had learned to feed on pellets showed food search and manipulation responses to a pellet-like pebble without smell and taste. -

3Cda99c90f15b1ffaba68178fdbd
A new insight to biomarkers related to resistance in survived-white spot syndrome virus challenged giant tiger shrimp, Penaeus monodon Farhana Mohd Ghani1,* and Subha Bhassu1,2,* 1 Department of Genetics & Molecular Biology, Institute of Biological Sciences, Faculty of Science, University of Malaya, Kuala Lumpur, Malaysia 2 Centre for Research in Biotechnology for Agriculture (CEBAR), University of Malaya, Kuala Lumpur, Malaysia * These authors contributed equally to this work. ABSTRACT The emergence of diseases such as white spot disease has become a threat to Penaeus monodon cultivation. Although there have been a few studies utilizing RNA-Seq, the cellular processes of host-virus interaction in this species remain mostly anonymous. In the present study, P. monodon was challenged with WSSV by intramuscular injection and survived for 12 days. The effect of the host gene expression by WSSV infection in the haemocytes,hepatopancreasandmuscleofP. monodonwasstudiedusingIlluminaHiSeq 2000. The RNA-Seq of cDNA libraries was developed from surviving WSSV-challenged shrimp as well as from normal healthy shrimp as control. A comparison of the transcriptome data of the two groups showed 2,644 host genes to be significantly up-regulatedand2,194genessignificantlydown-regulatedasaresultoftheinfectionwith WSSV. Among the differentially expressed genes, our study discovered HMGB, TNFSF andc-JuninP. monodonasnewpotentialcandidategenesforfurtherinvestigationforthe development of potential disease resistance markers. Our study also provided significant data on the differential expression of genes in the survived WSSV infected P. monodon that will help to improve understanding of host-virus interactions in this species. Submitted 18 February 2019 Accepted 27 October 2019 Published 20 December 2019 Subjects Aquaculture, Fisheries and Fish Science, Bioinformatics, Food Science and Technology, Corresponding author Genomics, Marine Biology Subha Bhassu, Keywords Novel discovery gene transcripts, Survived WSSV challenged shrimps, P. -
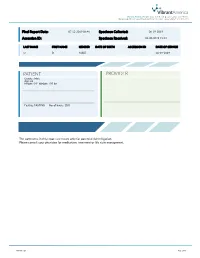
O-Labs-Summary Al
Vibrant America 1021 Howard Ave, Ste B. San Carlos, CA 94070 1(866) 364-0963 | [email protected] | www.vibrant-america.com Final Report Date: 07-12-2019 06:46 Specimen Collected: 06-19-2019 Accession ID: Specimen Received: 06-20-2019 15:53 LAST NAME FIRST NAME GENDER DATE OF BIRTH ACCESSION ID DATE OF SERVICE O B MALE 06-19-2019 PATIENT PROVIDER Gender: Male Age: 21 Height: 5'9'' Weight: 195 lbs Fasting: FASTING No. of hours: 10.0 The comments in this report are meant only for potential risk mitigation. Please consult your physician for medication, treatment or life style management. MK-0017-20 Page Page 1 1of of 6 47 Vibrant America | 1021 Howard Ave, Ste B. San Carlos, CA 94070 1(866) 364-0963 | [email protected] | www.vibrant-america.com PATIENT PROVIDER AGE: 21 HEIGHT: 5'9'' WEIGHT: 195 lbs P PHONE NUMBER: ADDRESS: ACCESSION ID: SPECIMEN COLLECTION TIME: 06-19-2019 SPECIMEN RECEIVED TIME: 06-20-2019 15:53 FINAL REPORT TIME: 07-12-2019 06:46 FASTING: FASTING NO. OF HOURS: 10.0 SUMMARY 1-4 L Low High Risk Moderate N/A - Not Ordered ALLERGEN SENSITIVITIES ALLERGEN SENSITIVITIES DIETARY ANTIGEN DIETARY ANTIGEN IgE (kU/L) IgG IgA IgE (kU/L) IgG IgA Alaska pollock - - - - Cherry L <0.10 L 2 L 2 Almond L <0.10 14 L 6 Chicken L <0.10 L 9 L 3 Amaranth L <0.10 L 2 L 1 Chickpea - - - - Anchovy - - - - Cinnamon L 0.26 L 2 L 3 Anise - - - - Clam L <0.10 L 5 L 3 Apple L <0.10 L 5 L 3 Cocoa L <0.10 L 1 L 1 Apricot L <0.10 13 20 Coconut L <0.10 L 3 L 3 Artichoke - - - - Codfish L <0.10 12 L 7 Asparagus - - - - Coffee L <0.10 14 -

Disease of Aquatic Organisms 100:211
Vol. 100: 211–218, 2012 DISEASES OF AQUATIC ORGANISMS Published September 12 doi: 10.3354/dao02496 Dis Aquat Org OPENPEN ACCESSCCESS Susceptibility of juvenile Macrobrachium rosenbergii to different doses of high and low virulence strains of white spot syndrome virus (WSSV) Mathias Corteel1, João J. Dantas-Lima1, Vo Van Tuan1, Khuong Van Thuong1, Mathieu Wille2, Victoria Alday-Sanz3, Maurice B. Pensaert1, Patrick Sorgeloos2, Hans J. Nauwynck1,* 1Laboratory of Virology, Faculty of Veterinary Medicine, Ghent University, 9820 Merelbeke, Belgium 2Laboratory of Aquaculture & Artemia Reference Center, Faculty of Bioscience Engineering, Ghent University, 9000 Gent, Belgium 3Pescanova, 08010 Barcelona, Spain ABSTRACT: As some literature on the susceptibility of different life stages of Macrobrachium rosenbergii to white spot syndrome virus (WSSV) is conflicting, the pathogenesis, infectivity and pathogenicity of 2 WSSV strains (Thai-1 and Viet) were investigated here in juveniles using con- ditions standardized for Penaeus vannamei. As with P. vannamei, juvenile M. rosenbergii (2 to 5 g) injected with a low dose of WSSV-Thai-1 or a high dose of WSSV-Viet developed comparable clin- ical pathology and numbers of infected cells within 1 to 2 d post-infection. In contrast, a low dose of WSSV-Viet capable of causing mortality in P. vannamei resulted in no detectable infection in M. −1 rosenbergii. Mean prawn infectious dose 50% endpoints (PID50 ml ) determined in M. rosen- 5.3±0.4 −1 bergii were in the order of 100-fold higher for WSSV-Thai-1 (10 PID50 ml ) than for WSSV- 3.2±0.2 −1 Viet (10 PID50 ml ), with each of these being about 20-fold and 400-fold lower, respectively, −1 than found previously in P. -

Penaeus Monodon) Promotoren: Prof
Haemocytic defence in black tiger shrimp (Penaeus monodon) Promotoren: Prof. dr. E. A. Huisman Hoogleraar in de Visteelt en Visserij Prof. dr. W. B. van Muiswinkel Hoogleraar in de Celbiologie en Immunologie Co-promotoren: Dr. W. P. W. van der Knaap Toegevoegd onderzoeker, Leerstoelgroep Visteelt en Visserij Dr. J. H. W. M. Rombout Universitair hoofddocent, Leerstoelgroep Celbiologie en Immunologie Overige leden promotiecommissie: Prof. dr. R. W. Goldbach, Wageningen Universiteit Dr. E. O. Rijke, Intervet International BV, Boxmeer Prof. dr. T. Sminia, Vrije Universiteit, Amsterdam Dr. V. J. Smith, University of St. Andrews, Scotland, UK Haemocytic defence in black tiger shrimp (Penaeus monodon) Karin van de Braak Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, prof. dr. ir. L. Speelman, in het openbaar te verdedigen op woensdag 5 juni 2002 des namiddags te vier uur in de aula. Haemocytic defence in black tiger shrimp (Penaeus monodon) PhD thesis, Wageningen University – with ref. – with summary in Dutch ISBN 90-5808-651-8 C. B. T. van de Braak, 2002 Wageningen Institute of Animal Sciences PO Box 338, 6700 AH Wageningen, The Netherlands Aan mijn ouders Ter nagedachtenis aan Jan Boon 5h gi Tropical shrimp culture is highly affected by infectious pathogens and disease control is nowadays a priority. The defence mechanisms of crustaceans are poorly understood, but knowledge of these is a prerequisite for the development of intervention strategies. Therefore, the aim of this thesis was to obtain a better understanding of the cellular defence system of the major cultured shrimp species, the black tiger shrimp (Penaeus monodon). -

Specific Molecular Signatures for Type II Crustins in Penaeid Shrimp
marine drugs Article Specific Molecular Signatures for Type II Crustins in Penaeid Shrimp Uncovered by the Identification of Crustin-Like Antimicrobial Peptides in Litopenaeus vannamei Cairé Barreto 1, Jaqueline da Rosa Coelho 1, Jianbo Yuan 2, Jianhai Xiang 2, Luciane Maria Perazzolo 1 and Rafael Diego Rosa 1,* ID 1 Laboratory of Immunology Applied to Aquaculture, Department of Cell Biology, Embryology and Genetics, Federal University of Santa Catarina, Florianópolis 88040-900 SC, Brazil; [email protected] (C.B.); [email protected] (J.d.R.C.); [email protected] (L.M.P.) 2 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China; [email protected] (J.Y.); [email protected] (J.X.) * Correspondence: [email protected]; Tel.: +55-48-37216163 Received: 30 August 2017; Accepted: 16 October 2017; Published: 16 January 2018 Abstract: Crustins form a large family of antimicrobial peptides (AMPs) in crustaceans composed of four sub-groups (Types I-IV). Type II crustins (Type IIa or “Crustins” and Type IIb or “Crustin-like”) possess a typical hydrophobic N-terminal region and are by far the most representative sub-group found in penaeid shrimp. To gain insight into the molecular diversity of Type II crustins in penaeids, we identified and characterized a Type IIb crustin in Litopenaeus vannamei (Crustin-like Lv) and compared Type II crustins at both molecular and transcriptional levels. Although L. vannamei Type II crustins (Crustin Lv and Crustin-like Lv) are encoded by separate genes, they showed a similar tissue distribution (hemocytes and gills) and transcriptional response to the shrimp pathogens Vibrio harveyi and White spot syndrome virus (WSSV). -

US Farmed Shrimp Production
Seafood Watch Seafood Report U.S. Farmed Shrimp Pacific white shrimp Litopenaeus vannamei © Sian Morgan/FishWise Final Report August 25, 2009 Victoria Galitzine, Siân Morgan, and Julio Harvey FishWise Seafood Watch/FishWise U.S. Farmed Shrimp Report August 25, 2009 About SFA®, Seafood Watch® and the Seafood Reports This report is a joint product of the Sustainable Fishery Advocates (SFA) and the Monterey Bay Aquarium Seafood Watch® program. Both organizations evaluate the ecological sustainability of wild-caught and farmed seafood commonly found in the United States marketplace. In doing so, SFA applies the definition of sustainable seafood and the method for its evaluation and presentation developed by the Seafood Watch program at the Monterey Bay Aquarium. Seafood Watch defines sustainable seafood as originating from species, whether wild-caught or farmed that can maintain or increase production into the long-term without jeopardizing the structure or function of affected ecosystems. SFA makes its sustainable seafood recommendations available to the public through these reports and its FishWise® program. FishWise® is a patented, educational program that provides information on sustainability, catch method, and origin of seafood found at retail outlets. The program seeks to educate consumers, restaurants, distributors, and retailers on sustainable fishery issues, with the goal of decreasing unsustainable fishing practices, while improving the livelihoods of people who fish, fish populations and ocean ecosystems. The body of this report synthesizes and evaluates current scientific information related to each of five sustainability criteria. For each criterion, research analysts at SFA seek out relevant scientific information from the following information sources (in order of preference): academic, peer-reviewed journals, government technical publications, fishery management plans and supporting documents, and other scientific reviews of ecological sustainability. -
Seafood Watch Seafood Report
Seafood Watch Seafood Report Wild-Caught Warmwater Shrimp (Family Penaeidae--the Penaeid shrimps) Image © Scandinavian Fishing Yearbook/www.scanfish.com Final Report April 26, 2004 Alice Cascorbi Fisheries Research Analyst About Seafood Watch® and the Seafood Reports Monterey Bay Aquarium’s Seafood Watch® program evaluates the ecological sustainability of wild-caught and farmed seafood commonly found in the United States marketplace. Seafood Watch® defines sustainable seafood as originating from sources, whether wild-caught or farmed, which can maintain or increase production in the long- term without jeopardizing the structure or function of affected ecosystems. Seafood Watch® makes its science-based recommendations available to the public in the form of regional pocket guides that can be downloaded from the Internet (seafoodwatch.org) or obtained from the Seafood Watch® program by emailing [email protected]. The program’s goals are to raise awareness of important ocean conservation issues and empower seafood consumers and businesses to make choices for healthy oceans. Each sustainability recommendation on the regional pocket guides is supported by a Seafood Report. Each report synthesizes and analyzes the most current ecological, fisheries and ecosystem science on a species, then evaluates this information against the program’s conservation ethic to arrive at a recommendation of “Best Choices”, “Good Alternatives” or “Avoid.” The detailed evaluation methodology is available upon request. In producing the Seafood Reports, Seafood Watch® seeks out research published in academic, peer-reviewed journals whenever possible. Other sources of information include government technical publications, fishery management plans and supporting documents, and other scientific reviews of ecological sustainability. Seafood Watch® Fisheries Research Analysts also communicate regularly with ecologists, fisheries and aquaculture scientists, and members of industry and conservation organizations when evaluating fisheries and aquaculture practices. -

Use of Soybean Meals in Diets of Omnivorous Freshwater Fishes
Use of Soybean Meal in the Diets of Marine Shrimp By Douglas E. Conklin, Department of Animal Science, University of California, Davis Written in cooperation with the United Soybean Board and American Soybean Association Introduction Depending on one’s focus, shrimp aquaculture either started as trapping and holding of wild seed (Ling et Shrimp aquaculture presently produces approximately al., 1977), or with the development of modern one million metric tons of shrimp annually. While some production techniques arising out of the research of 20 species are cultured in various parts of the world, the the Japanese scientist Motosaku Fujinaga (Fast, majority of production is based on eight species (Table 1992). The “trap and hold” approach requires little 1). For the eastern hemisphere, the fast growing giant effort on the part of the farmer, but yields are low tiger shrimp Penaeus monodon is the most important, and unpredictable. Traditionally with this type of while in the western hemisphere, the white shrimp approach, the shrimp feed and grow on available Litopenaeus vannamei is the leading production pond organisms. In some cases where additional species. seed is sourced from the wild, supplementary feed may be added to the pond. Availability of seed is Shrimp have a complicated life cycle (Figure 1). often the limiting factor for shrimp farmers using the Eggs from the female are broadcast into the marine “trap and hold” approach. environment. Hatching from the egg, the larvae pass through three distinct stages, nauplius, zoea and This bottleneck was partially bypassed by Fujinaga’s mysis, before assuming the distinctive adult development of methods allowing captive morphology as post-larval or juvenile shrimp.