The GAVI Alliance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gavi's Vaccine Investment Strategy
Gavi’s Vaccine Investment Strategy Deepali Patel THIRD WHO CONSULTATION ON GLOBAL ACTION PLAN FOR INFLUENZA VACCINES (GAP III) Geneva, Switzerland, 15-16 November 2016 www.gavi.org Vaccine Investment Strategy (VIS) Evidence-based approach to identifying new vaccine priorities for Gavi support Strategic investment Conducted every 5 years decision-making (rather than first-come- first-serve) Transparent methodology Consultations and Predictability of Gavi independent expert advice programmes for long- term planning by Analytical review of governments, industry evidence and modelling and donors 2 VIS is aligned with Gavi’s strategic cycle and replenishment 2011-2015 Strategic 2016-2020 Strategic 2021-2025 period period 2008 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 RTS,S pilot funding decision VIS #1 VIS #2 VIS #3 MenA, YF mass campaigns, JE, HPV Cholera stockpile, Mid 2017 : vaccine ‘long list’ Rubella, Rabies/cholera studies, Oct 2017 : methodology Typhoid Malaria – deferred Jun 2018 : vaccine shortlist conjugate Dec 2018 : investment decisions 3 VIS process Develop Collect data Develop in-depth methodology and Apply decision investment decision framework for cases for framework with comparative shortlisted evaluation analysis vaccines criteria Phase I Narrow long list Phase II Recommend for Identify long list to higher priority Gavi Board of vaccines vaccines approval of selected vaccines Stakeholder consultations and independent expert review 4 Evaluation criteria (VIS #2 – 2013) Additional Health Implementation -

CLINICAL TRIALS Safety and Immunogenicity of a Nicotine Conjugate Vaccine in Current Smokers
CLINICAL TRIALS Safety and immunogenicity of a nicotine conjugate vaccine in current smokers Immunotherapy is a novel potential treatment for nicotine addiction. The aim of this study was to assess the safety and immunogenicity of a nicotine conjugate vaccine, NicVAX, and its effects on smoking behavior. were recruited for a noncessation treatment study and assigned to 1 of 3 doses of the (68 ؍ Smokers (N nicotine vaccine (50, 100, or 200 g) or placebo. They were injected on days 0, 28, 56, and 182 and monitored for a period of 38 weeks. Results showed that the nicotine vaccine was safe and well tolerated. Vaccine immunogenicity was dose-related (P < .001), with the highest dose eliciting antibody concentrations within the anticipated range of efficacy. There was no evidence of compensatory smoking or precipitation of nicotine withdrawal with the nicotine vaccine. The 30-day abstinence rate was significantly different across with the highest rate of abstinence occurring with 200 g. The nicotine vaccine appears ,(02. ؍ the 4 doses (P to be a promising medication for tobacco dependence. (Clin Pharmacol Ther 2005;78:456-67.) Dorothy K. Hatsukami, PhD, Stephen Rennard, MD, Douglas Jorenby, PhD, Michael Fiore, MD, MPH, Joseph Koopmeiners, Arjen de Vos, MD, PhD, Gary Horwith, MD, and Paul R. Pentel, MD Minneapolis, Minn, Omaha, Neb, Madison, Wis, and Rockville, Md Surveys show that, although about 41% of smokers apy, is about 25% on average.2 Moreover, these per- make a quit attempt each year, less than 5% of smokers centages most likely exaggerate the efficacy of are successful at remaining abstinent for 3 months to a intervention because these trials are typically composed year.1 Smokers seeking available behavioral and phar- of subjects who are highly motivated to quit and who macologic therapies can enhance successful quit rates are free of complicating diagnoses such as depression 2 by 2- to 3-fold over control conditions. -

Yellow Fever 2016
Resident / Humanitarian Coordinator Report on the use of CERF funds RESIDENT / HUMANITARIAN COORDINATOR REPORT ON THE USE OF CERF FUNDS ANGOLA RAPID RESPONSE YELLOW FEVER 2016 RESIDENT/HUMANITARIAN COORDINATOR Pier Paolo Balladelli REPORTING PROCESS AND CONSULTATION SUMMARY a. Please indicate when the After Action Review (AAR) was conducted and who participated. Review agreed on 02/09/2016 and 07/09/2016. b. Please confirm that the Resident Coordinator and/or Humanitarian Coordinator (RC/HC) Report was discussed in the Humanitarian and/or UN Country Team and by cluster/sector coordinators as outlined in the guidelines. YES NO c. Was the final version of the RC/HC Report shared for review with in-country stakeholders as recommended in the guidelines (i.e. the CERF recipient agencies and their implementing partners, cluster/sector coordinators and members and relevant government counterparts)? YES NO Final version shared with UNICEF and UNDP, although this initiative was mainly implemented by WHO 2 I. HUMANITARIAN CONTEXT TABLE 1: EMERGENCY ALLOCATION OVERVIEW (US$) Total amount required for the humanitarian response: Source Amount CERF 3,000,000 Breakdown of total response COUNTRY-BASED POOL FUND (if applicable) 4,508,559 funding received by source OTHER (bilateral/multilateral) TOTAL 10,473,618 *The total amount does not match because this was considered an underfunded emergency. TABLE 2: CERF EMERGENCY FUNDING BY ALLOCATION AND PROJECT (US$) Allocation 1 – date of official submission: 06/04/2016- 05/10/2016 Agency Project code Cluster/Sector -

Tuesday, September 24, 2019 Time: 6:00Pm – 8:00Pm Location: Delegates Dining Room (Private Dining Rooms 6 – 8) Please Email [email protected] to RSVP
The World Health Organization, the United Nations Development Programme and the Global Fund are pleased to invite you to a Side-Event to the High-Level Meeting on Universal Health Coverage: Anti-Corruption, Transparency and Accountability in Health. A multi-sectoral panel discussion will explore the impact of corruption on achieving Sustainable Development Goal (SDG) 3, ensuring healthy lives and promoting wellbeing for all, with a specific focus on SDG 3.8, achieving universal health coverage (UHC). The discussion will consider how the ongoing establishment of an Anti-Corruption, Transparency and Accountability for Health Alliance (ACTA) can leverage its multi-stakeholder platform to foster coordination and collaboration, provide normative guidance to assist countries in developing strategies and partnerships to prevent health sector corruption, and contribute to interventions that strengthen the global pledge to leave no one behind. Date: Tuesday, September 24, 2019 Time: 6:00pm – 8:00pm Location: Delegates Dining Room (Private Dining Rooms 6 – 8) Please email [email protected] to RSVP. Due to limited seating, please confirm your attendance no later than Thursday, 19 September 2019. AGNÉS SOUCAT Director for Health Systems, Governance and Financing World Health Organization MANDEEP DHALIWAL DR. PETER SALAMA MARIJKE WIJNROKS Director of HIV, Health and Executive Director, Universal Health Coverage Chief of Staff Development Practice Life Course The Global Fund United Nations Development Programme World Health Organization JILLIAN KOHLER ALLAN MALECHE Professor, Leslie Dan Faculty of Pharmacy Executive Director University of Toronto KELIN DIWA SAMAD WILLIAM SAVEDOFF Deputy Minister of Policy and Planning Senior Fellow Ministry of Public Health Center for Global Development Afghanistan Mandeep Dhaliwal Director, HIV, Health and Development Team, Bureau of Policy and Programme Support United Nations Development Programme Mandeep Dhaliwal is the Director of UNDP’s HIV, Health and Development Group, Bureau of Policy and Programme Support. -

Hanna Nohynek @Hnohynek
Biosketch Hanna Nohynek @hnohynek Hanna Nohynek is Chief Physician and Deputy Head of the Infectious Diseases Control and Vaccines Unit of the Department of Health Security at the Finnish Institute for Health and Welfare. She serves as secretary of the Finnish NITAG (KRAR), and leads the subgroup on Strategic development of the influenza vaccination programme and the subgroup on the SARS-CoV-2 vaccination strategy. She practices clinical medicine at a travel health clinic in Aava, Helsinki. She was instrumental in designing the first THL (KTL) health advisory for refugees and asylum seekers in Finland, studying the narcolepsy signal post pandemic vaccination, designing the introduction of the HPV vaccine to the national immunization programme, and the introduction of the live attenuated influenza vaccine for children. Her present research interests are register-based vaccine impact studies, evidence based policy/decision making, vaccine safety, hesitancy, SARS-CoV-2, RSV, influenza and pneumococcus. She coordinates the work packages on field studies and communication for IMI DRIVE on brand specific influenza vaccine effectiveness (www.drive-eu.org). She has authored more than 130 original articles (including the first scientific report on the association between pandemic influenza vaccination and narcolepsy), and she teaches, giving over 30 invited lectures annually and guiding elective, graduate and PhD students (presently Raija Auvinen and Idil Hussein). She belongs to the external faculty of the University of Tampere MSc course on Global Health. She has served on expert committees evaluating HBV, PCV and rota virus vaccines in Finland, and as an advisor to the EU, IMI, IVI, WHO, GAVI, SIDA/SRC, and the Finnish MOFA. -

Bulletin of the World Health Organization
News WHO’s new emergencies programme bridges two worlds Peter Salama tells Fiona Fleck how the World Health Organization’s (WHO) new emergencies programme is changing the way the agency helps countries prepare for and respond to health crises. Q: WHO’s new health emergencies programme aims to create one single Peter Salama is leading the World Health Organization’s programme, with one workforce, one (WHO) efforts to reform its emergency work. He was budget, one set of rules and processes appointed Executive Director of WHO’s new Health and one clear line of authority. How Emergencies Programme at the level of Deputy will you do this with WHO’s governance Director-General last year. Before that, he held senior structure of seven entities: headquarters posts at the United Nations Children’s Fund (UNICEF) (HQ) plus six Regional Offices? including Regional Director for the Middle East and A: The programme has one work- North Africa, Global Coordinator for Ebola and Chief of force – and that is the critical point – one WHO set of people we can rely on in emergen- Peter Salama Global Health. Before joining UNICEF in 2002, Salama cies, whether they are at regional level was an epidemic intelligence service officer at the or at HQ, and, increasingly, we want International Emergency and Refugee Health Branch of the United States the heads of Country Offices to see Centers for Disease Control and Prevention, and a visiting professor in nutrition themselves as an integral part of the at Tufts University in the United States of America. He has worked with Médecins programme with the same philosophy, Sans Frontières and Concern Worldwide in Asia and sub-Saharan Africa. -

Ebola: Democratic Republic of Congo
CRS INSIGHT Ebola: Democratic Republic of Congo Updated October 4, 2018 (IN10917) | Related Author Tiaji Salaam-Blyther | Tiaji Salaam-Blyther, Coordinator, Specialist in Global Health ([email protected], 7-7677) Monyai L. Chavers, Research Assistant ([email protected], 7-0829) On August 1, 2018, the World Health Organization (WHO) reported that a new Ebola outbreak was detected in the eastern part of the Democratic Republic of Congo (DRC), about one week after having declared that a separate outbreak had ended in the western part of the country. This new outbreak is occurring in North Kivu and Ituri provinces, the most populated provinces in DRC, where a humanitarian crisis affecting over 1 million displaced people is ongoing. Health workers have begun vaccinating people in the districts to control the spread of the disease, though armed conflict in the areas is complicating control efforts. As of October 2, 2018, 162 people have contracted Ebola in North Kivu and Ituri provinces (including 19 health workers), 106 of whom have died (3 of whom were health workers). This outbreak is the 10th Ebola outbreak in DRC since the disease was discovered in 1976, and it stands in stark contrast to the previous outbreak (Figure 1). The outbreak that began in May 2018 was contained and ended within two months after having infected 54 people, including 33 of whom died. In its second month, this outbreak has caused twice as many deaths and is continuing to spread. Issues complicating efforts to contain the current outbreak include the following: Conflict. In September 2018, clashes between rebels and government forces had forced WHO to temporarily suspend operations in Beni, the WHO operational base. -

UNEP Mercury Treaty Protects Access to Thiomersal-Containing Vaccines
UNEP mercury treaty protects access to thiomersal-containing vaccines United Nations Environment Program has developed a treaty on mercury in an effort to protect human health and the environment by limiting mercury releases. In the course of the negotiations, a proposal was made to restrict vaccines that contain the preservative thiomersal under a section of the treaty that prohibits trade of mercury-added products. The implications of restricting thiomersal, an ethyl mercury-containing preservative, would be significant. According to SAGE, “Thiomersal-containing vaccines [are] safe, essential, and irreplaceable components of immunization programs, especially in developing countries, and…removal of these products would disproportionately jeopardize the health and lives of the most disadvantaged children worldwide.” The treaty annex that describes prohibited products specifically excludes “vaccines containing thiomersal as preservatives” under a short list of products the authors intended to emphasize were to be protected. Protecting access to vaccines came as the result of a strong partnership between WHO, UNICEF, GAVI, and civil society advocates and experts around the world to educate country delegates, who predominantly came from ministries of environment. This was also a wonderful partnership with animal health experts, who similarly rely on thiomersal for veterinary vaccines. By facilitating communication between ministries of health and ministries of environment, strong statements are made by delegates about the essential role of thiomersal-containing vaccines in protecting human health. PATH will be collecting and disseminating additional information about how the community came together around this issue and lessons learned in the coming months. . -

Immunization Policies and Procedures Manual
Immunization Policies and Procedures Manual Louisiana Department of Health Office of Public Health Immunization Program Revised September 2017 i Center for Community and Preventive Health Bureau of Infectious Diseases Immunization Program TABLE OF CONTENTS I. POLICY AND GENERAL CLINIC POLICY ............................................................................................................................. 1 PURPOSE ........................................................................................................................................................................................... 1 POLICY ON CLINIC SCHEDULING ............................................................................................................................................ 2 POLICY ON PUBLICITY FOR IMMUNIZATION ACTIVITIES .............................................................................................. 4 POLICY ON EDUCATIONAL ACTIVITIES (HEALTH EDUCATION IN IMMUNIZATION CLINICS) .......................... 5 POLICY ON CHECKING IMMUNIZATION STATUS OF ALL CHILDREN RECEIVING SERVICES THROUGH THE HEALTH DEPARTMENT ...................................................................................................................................................... 6 POLICY ON MAXIMIZING TIME SPENT WITH PARENTS DURING IMMUNIZATION CLINICS ............................... 7 POLICY ON ASSISTANCE TO FOREIGN TRAVELERS .......................................................................................................... 9 II. POLICY -

UNICEF Immunization Roadmap 2018-2030
UNICEF IMMUNIZATION ROADMAP 2018–2030 Cover: ©UNICEF/UN065768/Khouder Al-Issa A health worker vaccinates 3-year-old Rahaf in Tareek Albab neighborhood in the eastern part of Aleppo city. UNICEF IMMUNIZATION ROADMAP 2018–2030 Photograph credits: Pages vi-vii: © Shutterstock/thi Page 5: © UNICEF/UN060913/Al-Issa Page 6: © UNICEF/UNI41364/Estey Page 10: © UNICEF/UN061432/Dejongh Page 14: © UNICEF/UNI76541/Holmes Page 18: © UNICEF/UN0125829/Sharma Page 24: © UNICEF/UN059884/Zar Mon Page 26: © UNICEF/UN065770/Al-Issa Page 33: © UNICEF/UN0143438/Alhariri Page 34: © UNICEF/UNI45693/Estey Page 38: © UNICEF/UNI77040/Holmes Page 40: © UNICEF/UN0125861/Sharma Page 44: © UNICEF/UNI41211/Holmes © United Nations Children’s Fund (UNICEF), September 2018 Permission is required to reproduce any part of this publication. Permission will be freely granted to educational or non-profit organizations. Please contact: Health Section, Immunization Team UNICEF 3 United Nations Plaza New York, NY 100017 Contents Abbreviations viii Executive summary 1 1. Introduction 7 1.1 Background 7 1.2 Developing the Roadmap 9 2. Key considerations informing the UNICEF Immunization Roadmap 11 2.1 Key drivers of immunization through 2030 11 2.2 Evolving immunization partnerships 15 2.3 UNICEF’s comparative advantage in immunization 16 3. What is new in this Roadmap? 19 3.1 Immunization coverage as a tracer indicator of child equity 19 3.2 Key areas of work 21 4. Roadmap programming framework 27 4.1 Vision and impact statements 27 4.2 Programming principles 27 4.3 Context-driven responses 27 4.4 Objectives and priorities 29 4.5 Populations and platforms 32 4.6 Country-level immunization strategies 32 5. -
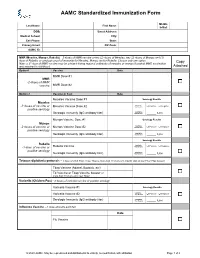
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
AAMC Standardized Immunization Form 2020 Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC) 1. What are the hepatitis B vaccines licensed for use in the United States? Three single-antigen vaccines and two combination vaccines are currently licensed in the United States. Single-antigen hepatitis B vaccines: • ENGERIX-B® • RECOMBIVAX HB® • HEPLISAV-B™ Combination vaccines: • PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years. • TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections. 2. What are the recommended schedules for hepatitis B vaccination? The vaccination schedule most often used for children and adults is three doses given at 0, 1, and 6 months. Alternate schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved to be given as two doses one month apart. 3. If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted? No. The series does not need to be restarted but the following should be considered: • If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible. • The second and third doses should be separated by an interval of at least 8 weeks. • If only the third dose is delayed, it should be administered as soon as possible. 4. Is it harmful to administer an extra dose of hepatitis B vaccine or to repeat the entire vaccine series if documentation of the vaccination history is unavailable or the serology test is negative? No, administering extra doses of single-antigen hepatitis B vaccine is not harmful.