Injection Augmentation and Endoscopic Repair of Type 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
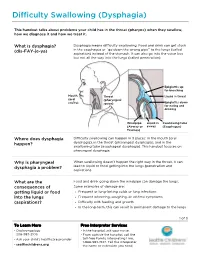
PE3334 Difficulty Swallowing (Dysphagia)
Difficulty Swallowing (Dysphagia) This handout talks about problems your child has in the throat (pharynx) when they swallow, how we diagnose it and how we treat it. What is dysphagia? Dysphagia means difficulty swallowing. Food and drink can get stuck (dis-FAY-je-ya) in the esophagus or “go down the wrong pipe” to the lungs (called aspiration) instead of the stomach. It can also go into the voice box but not all the way into the lungs (called penetration). Epiglottis up for breathing Mouth Throat Liquid in throat (oral (pharyngeal cavity) space) Epiglottis down for eating and drinking Windpipe Liquid in Swallowing tube (Airway or airway (Esophagus) Trachea) Where does dysphagia Difficulty swallowing can happen in 3 places: in the mouth (oral happen? dysphagia), in the throat (pharyngeal dysphagia), and in the swallowing tube (esophageal dysphagia). This handout focuses on pharyngeal dysphagia. Why is pharyngeal When swallowing doesn’t happen the right way in the throat, it can dysphagia a problem? lead to liquid or food getting into the lungs (penetration and aspiration). What are the Food and drink going down the windpipe can damage the lungs. consequences of Some examples of damage are: getting liquid or food • Frequent or long-lasting colds or lung infections into the lungs • Frequent wheezing, coughing, or asthma symptoms (aspiration)? • Difficulty with feeding and growth • In the long-term, this can result in permanent damage to the lungs 1 of 3 To Learn More Free Interpreter Services • Otolaryngology • In the hospital, ask your nurse. 206-987-2105 • From outside the hospital, call the • Ask your child’s healthcare provider toll-free Family Interpreting Line, 1-866-583-1527. -

Laryngeal Clefts- Associated Syndromes and Management
[NM-295] Laryngeal clefts- Associated syndromes and Management Tran L, Flotildes K Children's Hospital Los Angeles , Los Angeles , CA, USA Laryngeal clefts are congenital anomalies of the posterior larynx forming a communication between the trachea and esophagus. The incidence of laryngeal clefts is rare, occurring 1 in 10,000 to 20,000 births. We present two patients with laryngeal clefts, one with a mild and the other with a severe form. Patient A was a 4 week old 3.8 kg baby girl born full term with cleft lip, cleft palate, atrial septal defect and ventricular septal defect who presented with coughing during feeds. Imaging showed contrast traveling from the esophagus into the anterior trachea. Direct laryngoscopy and bronchoscopy with spontaneous ventilation revealed an H-type tracheoesophageal fistula and a grade 1 laryngeal cleft. The patient underwent a tracheoesophageal fistula ligation and conservative management was recommended for the laryngeal cleft. Opitz G was considered as a possible diagnosis for the patient’s syndrome. Patient B was a 19 day old 1.1kg born at 29 weeks who presented with respiratory distress. The patient was intubated at birth and had recurrent desaturations and bradycardia when extubated. Direct laryngoscopy and bronchoscopy were performed at bedside instead of the operating room due to desaturation with movement of the endotracheal tube. The patient was found to have a grade 3 laryngeal cleft which required surgical repair with ECMO or cardiopulmonary bypass. Due to the patient's size, prematurity, and guarded prognosis, the family decided to withdraw care. Laryngeal clefts account for 0.2 to 1.5% of congenital airway anomalies and can be associated with syndromes such as Opitz G and Pallister Hall. -
7.1 Birth Defects Code List
7.1 BIRTH DEFECTS CODE LIST Based on the British Pediatric Association (BPA) Classification of Diseases (1979) and the World Health Organization's International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) (1979) Code modifications developed by Division of Birth Defects and Developmental Disabilities National Center on Birth Defects and Developmental Disabilities Centers for Disease Control and Prevention Public Health Service U.S. Department of Health and Human Services Atlanta, Georgia 30333 and Birth Defects Epidemiology and Surveillance Branch Epidemiology and Disease Surveillance Unit Texas Department of State Health Services 1100 West 49th Street Austin, TX 78756 Revised July 31, 2019 TABLE OF CONTENTS Table of Contents .................................................................................................................................................. 2 Introduction To Birth Defect Diagnosis Coding ................................................................................................. 7 Purpose ............................................................................................................................................................ 7 BPA coding system .......................................................................................................................................... 7 Description of the BPA code ............................................................................................................................ 8 Problems with the -

ICD-10 Coding Manual List of All Reportable Congenital Malformations
New York State Department of Health Congenital Malformations Registry ICD-10 Coding Manual List of all Reportable Congenital Malformations Last updated 10/22/2019 - 1 - _________________________________________________________________________ Table of Contents Reporting Requirements and Instructions ............................................................................ - 3 - Children to Report: ............................................................................................................ - 3 - What to Report: ................................................................................................................. - 3 - Common Acronyms: ......................................................................................................... - 3 - Color Coding: .................................................................................................................... - 3 - Common Notation: ............................................................................................................ - 3 - Congenital Malformations of the Nervous System (Q00-Q07) .............................................. - 4 - Congenital Malformations of Eye, Ear, Face and Neck (Q10-Q18) .................................... - 11 - Congenital Malformations of the Circulatory System (Q20-Q28) ........................................ - 17 - Congenital Malformations of the Respiratory System (Q30-Q34) ....................................... - 24 - Congenital Malformations of the Cleft Lip and Cleft Palate (Q35-Q37) ............................. -

Management of a Case of Laryngotracheal Esophageal Cleft Type III with Review of Literature
Pediatric Anesthesia and Critical Care Journal 2015; 3(2):80-84 doi:10.14587/paccj.2015.15 Management of a case of laryngotracheal esophageal cleft type III with review of literature U. Badole, S. D. Mundada, S. Patil, A. Rathod Department of Anaesthesia and Critical Care, Sir J. J. Group of Hospitals, Mumbai India Corresponding author: S. D. Mundada, Department of Anaesthesia and Critical Care, Sir J. J. Group of Hospitals, Mumbai, India. Email: [email protected] Key points Laryngotracheal esophageal cleft (LTEC) is a rare developmental disorder of upper airway which accounts for 0.3% to 0.5% of all congenital anomalies of the larynx. It demands high clinical judgment to diagnose the case as most of the times patient is erroneously diagnosed as tracheoesophageal fistula and recurrent esophageal intubation or slipping of endotacheal tube in the esophagus should arise suspicion of LTEC. It has ben divided into four types by Benjamin and Inglis and severity depends upon type. Type III and IV present with severe symptoms in the neonatal period. Know- ledge of different approaches to secure and maintain airway and preparedness for the same in such a case is most im- portant to prevent catastrophic events. Abstract The trachea and esophagus originate from one common Laryngotracheal esophageal cleft (LTEC) is an abnor- tube and are separated by the rostral development of the mal communication of larynx and trachea with esopha- tracheoesophageal septum, which is complete at appro- gus via a midline defect. This anomaly occurs in less ximately 35 days of gestation. Complete or partial failu- than 1% of population, and has slight male prelidection. -

Laryngeal Cleft NON-ORAL FEEDINGS NON-ORAL FEEDINGS
FEEDING THE MEDICAL COMPLEX PEDIATRIC PATIENT Brenda Sitzmann, MA, CCC-SLP [email protected] (816) 302-8037 DISCLOSURES ➤ Ms. Sitzmann is speech-language pathologist at Children’s Mercy for which she receives a salary. ➤ Ms. Sitzmann is receiving an honorarium for presenting this workshop. ➤ Ms. Sitzmann has no non-financial relationships to disclose. PEDIATRIC FEEDING DISORDER Goday, et al. 2017 ➤ An impairment (system failure) creates a pediatric feeding disorder (PFD) that results in a dysfunction ➤ The resulting dysfunction is not considered age- appropriate ➤ Impacts at least one of these domains: ➤ medical ➤ nutrition ➤ skill and ability ➤ psychosocial ➤ Today’s presentation will focus on the impact of the medical domain on skill and ability. OVERVIEW ➤ Non-oral feedings ➤ Eosinophilic esophagitis ➤ Laryngomalacia ➤ Laryngeal cleft NON-ORAL FEEDINGS NON-ORAL FEEDINGS ➤ Very useful tools ➤ Enables patients to receive adequate nutrition while the team addresses medical, psychosocial, and skill/ability concerns ➤ Have a plan to work towards removal before the tube is placed however this may not be the goal for all patients NON-ORAL FEEDINGS Studies have shown that g-tubes: ➤ Improve weight gain and mid-arm circumference ➤ Reduce feeding time ➤ Improve quality of life for caregivers and patients (Gottrand et al. 2010) NON-ORAL FEEDINGS Today we are going to explore the following types of non-oral feedings: ➤ Nasogastric (NG) tube ➤ Nasojejunal (NJ) tube ➤ Gastrostomy (G) tube ➤ Gastro-jejunal (G/J) tube NASOGASTRIC (NG) TUBE ➤ A long -
Type 1 Laryngeal Cleft a Multidimensional Management Algorithm
Research Original Investigation Type 1 Laryngeal Cleft A Multidimensional Management Algorithm Shilpa Ojha, MBChB, MRCS; Jean E. Ashland, PhD, CCC-SLP; Cheryl Hersh, MA, CCC-SLP; Jyoti Ramakrishna, MD; Rie Maurer, MA; Christopher J. Hartnick, MD CME Quiz at jamanetworkcme.com and IMPORTANCE Early diagnosis and assessment in children with type 1 laryngeal cleft are CME Questions page 84 essential in preventing aspiration and associated comorbidity. Appropriate use of conservative and surgical interventions in an evidence-based management strategy can improve overall outcome. OBJECTIVE To evaluate the management of care for children with type 1 laryngeal cleft in our practice and develop an updated management algorithm. DESIGN, SETTING, AND PARTICIPANTS We performed a review of medical records at a tertiary pediatric aerodigestive center. During a period of 7 years (July 18, 2005, to July 18, 2012), 1014 children younger than 18 years were evaluated for aspiration, choking, cough, or recurrent pneumonia. Of these, 44 children (4.3%) had a type 1 laryngeal cleft. Two were lost to follow-up; thus, 42 children were included in our final sample (28 males, 14 females). INTERVENTIONS The care of 15 patients (36%) was managed conservatively, and 27 patients (64%) underwent endoscopic surgical repair of their laryngeal cleft. MAIN OUTCOME AND MEASURE Assessment of our current management strategy. RESULTS Success was defined as improving when a child was able to tolerate a feeding without aspirating or resolved when the child had transitioned to tolerating thin liquids. All patients received a trial of conservative therapy. Fifteen of the 42 patients (36%) had an anatomic cleft and were able to maintain the feeding regimen; thus, conservative treatment was successful in this group. -
Laryngo-Tracheo-Oesophageal Clefts Nicolas Leboulanger* and Eréa-Noël Garabédian
Leboulanger and Garabédian Orphanet Journal of Rare Diseases 2011, 6:81 http://www.ojrd.com/content/6/1/81 REVIEW Open Access Laryngo-tracheo-oesophageal clefts Nicolas Leboulanger* and Eréa-Noël Garabédian Abstract A laryngo-tracheo-esophageal cleft (LC) is a congenital malformation characterized by an abnormal, posterior, sagittal communication between the larynx and the pharynx, possibly extending downward between the trachea and the esophagus. The estimated annual incidence of LC is 1/10,000 to 1/20,000 live births, accounting for 0.2% to 1.5% of congenital malformations of the larynx. These incidence rates may however be underestimated due to difficulty in diagnosing minor forms and a high mortality rate in severe forms. A slightly higher incidence has been reported in boys than in girls. No specific geographic distribution has been found. Depending on the severity of the malformation, patients may present with stridor, hoarse cry, swallowing difficulties, aspirations, cough, dyspnea and cyanosis through to early respiratory distress. Five types of laryngo-tracheo-esophageal cleft have been described based on the downward extension of the cleft, which typically correlates with the severity of symptoms: Type 0 laryngo-tracheo- esophageal cleft to Type 4 laryngo-tracheo-esophageal cleft. LC is often associated with other congenital abnormalities/ anomalies (16% to 68%), mainly involving the gastro-intestinal tract, which include laryngomalacia, tracheo-bronchial dyskinesia, tracheo-bronchomalacia (mostly in types 3 and 4), and gastro-esophageal reflux disease (GERD). The syndromes most frequently associated with an LC are Opitz/BBB syndrome, Pallister Hall syndrome, VACTERL/VATER association, and CHARGE syndrome. Laryngeal clefts result from failure of fusion of the posterior cricoid lamina and abnormal development of the tracheo-esophageal septum. -

An Aerodigestive Perspective
11/1/19 “Why Does My Child Cough So Much?”: An Aerodigestive Perspective Elton Lambert, MD Eric Chiou, MD Julie P. Katkin MD Catherine L. Turk, PhD, CCC-SLP Aerodigestive Program at Texas Children’s Hospital 81 ObJectives • Development of schema for evaluating patients with chronic cough • Multidisciplinary evaluation of a child with a chronic cough and feeding difficulties – Otolaryngology – Gastroenterology – Pulmonology – Speech Pathology • Role of triple endoscopy and swallowing evaluations in a child with a chronic cough and chronic respiratory complaints 82 1 11/1/19 Chronic Cough A cough of more than 3 to 4 weeks duration Sadof M, Kasloivsky, R Pediatrics in Review November 2013, VOLUME 34 / ISSUE 11 Chronic Cough in Children: A Primary Care and Subspecialty Collaborative Approach 83 Chronic Cough A cough of more than 3 to 4 weeks duration 84 2 11/1/19 Chronic Cough A cough of more than 3 to 4 weeks duration 85 Chronic Cough A cough of more than 3 to 4 weeks duration 86 3 11/1/19 Case Why does my child cough so much? 87 Why does my child cough so much? • 11 month old, former 36 weeker • Noisy breathing and one episode of croup • Wet cough, frequent respiratory infections and “asthma” • Spitting up and “reflux” • Choking and gagging on feeds 88 4 11/1/19 From an Otolaryngologist’s Perspective Noisy Breathing – Squeak, Snore or Gurgle • Stridor • Stertor & Snoring – Inspiratory • Gurgling – Expiratory – Secretion Handling – Biphasic 89 From an Otolaryngologist’s Perspective Noisy BreathingBreathing – –Squeak,Squeak, Snore Snore or -

Laryngeal Cleft—Case Series from a Surgical Neonatal Intensive Care Unit
Original Article Page 1 of 6 Laryngeal cleft—case series from a surgical neonatal intensive care unit Christine Jorgensen, Amit Trivedi, Alan Cheng, Jonathan De Lima, Karen Walker The Children’s Hospital at Westmead, Discipline of Child and Adolescent Health, University of Sydney, Sydney, NSW, Australia Contributions: (I) Conception and design: C Jorgensen, A Trivedi, K Walker; (II) Administrative support: C Jorgensen; (III) Provision of study materials or patients: C Jorgensen, A Trivedi, K Walker; (IV) Collection and assembly of data: C Jorgensen; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Christine Jorgensen. Grace Centre for Newborn Care, the Children’s hospital at Westmead, Locked Bag 4001, Westmead, NSW 2145, Australia. Email: [email protected]. Background: Laryngeal clefts are rare congenital anomalies of the upper aerodigestive tract with a persistent connection between the posterior laryngotracheal airway and the oesophagus. The purpose of this study was to review the clinical presentation, management and outcomes of a cohort of infants presenting with laryngeal clefts to a tertiary surgical neonatal intensive care unit in the newborn period. Methods: A single centre retrospective case review was conducted on infants with a diagnosis of types I to IV laryngeal cleft. Results: Eight infants with laryngeal clefts were identified. The median age at presentation was 1.5 days (1–118 days) and median age to diagnosis was 6.5 days. Seven out of eight neonates were male and five were born prematurely. Seven out of eight had a significant comorbidity and four infants died. -

Type I Laryngeal Clefts: to Stitch Or Not to Stitch
Type I Laryngeal Clefts: To Stitch or Not to Stitch Paula J. Harmon, MD Emory University School of Medicine Assistant Professor Department of Otolaryngology/Head and Neck Surgery Division of Pediatric Otolaryngology Disclosures NONE Objectives Review anatomy of laryngeal clefts Current methods of repair of Type I Laryngeal clefts Benefits Drawbacks Review new techniques in endoscopic laryngeal repair Benefits Drawbacks History of Laryngeal Clefts Rare congenital anomaly Congenital laryngeal anomaly occurs in 1 in 2000 live births 0.3% are laryngeal clefts Boy:Girl… 5:3 ratio Associated with VACTERL, Opitz-Frias syndrome, Pallister-Hall syndrome Embryology of Larynx Respiratory primordium develop from diverticulum on foregut Tracheobronchial groove arise on either side and fuse in the midline and for tracheoesophageal septum Fusion complete in 6th week of gestation Cricoid cartilage forms 5th week Incomplete fusion of tracheoesophageal septum or cricoid cartilage result in laryngeal cleft/T-E fistula Laryngeal Development Laryngeal Clefts Benjamin and Inglis classification of laryngotracheal clefts Type Laryngotracheal Defect I Supraglottic interarytenoid defect; the level of the cleft remains above the level of the (true) vocal cord II The cleft extends below the level of the (true) vocal cords and partially into the cricoid cartilage III The cleft extends completely through the posterior cricoid cartilage, with or without further extension into the cervical tracheo-esophageal wall IV Common tracheo-esophagus that extends -

Laryngo-Tracheo-Oesophageal Cleft
532 Dodd DS. 3 Behrman E, Vaughan VC, eds. Jaundice and hyperbilrubinemia in the new- 17 Pearlman MA, Gartner LM, Lee K-S, Morecki R, Horoupian Arch Dis Child: first published as 10.1136/adc.68.5_Spec_No.532 on 1 May 1993. Downloaded from born. Nelson textbook of pediatics. 13th Ed. Philadelphia: WB Saunders, Absence of kernicterus in low-birth-weight infants from 1971 through 1987: 405-7. 1976: comparison with findings in 1966 and 1967. Pediatrics 1978; 62: 4 Oski FA. Physiologic jaundice. In: Avery ME, Taeusch HW Jr, eds. Diseases 460-4. ofthe newborn. 5th Ed. Philadelphia: WB Saunders, 1988: 625. 18 Valaes T, Gellis SS. Is kernicterus always the definitive evidence of bilirubin 5 Maisels MJ. Neonatal jaundice. In: Avery GB, ed. Neonataology, patho-phy- toxicity. Pediatrics 1981; 67: 940-1. isology and management ofthe newborn. 3rd Ed. Philadelphia: JB Lippincott, 19 Scheidt PC, Mellits ED, Hardy JB, et al. Toxicity to bilirubin in neonates; 1987: 534-629. infant development during the first year in relation to maximum neonatal 6 Maisels MJ, Gifford K. Norman serum bilirubin levels in the newborn and serum bilirubin concentration. J Pediatr 1977; 91: 292-7. the effect of breast-feeding. Pediatrics 1986; 78: 837-43. 20 Naeye RL. Amniotic fluid infections, neonatal hyperbilirubinemia and 7 Newman TB, Easterling MJ, Goldman ES, Stevenson DK. Laboratory eval- psychomotor impairment. Pediatrics 1978; 62: 497-503. uation of jaundiced newborns: frequency, cost and yield. Am J Dis Child 21 Corchia C, Ruiu M, Orzalesi M. Breast-feeding and hyperbilirubinema in 1990; 144: 364-8. full-term newborn infants.