Efficacy of Triclosan Soap Against Superficial Dermatomycoses and Scabies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Epidemiology of Dandruff, Scalp Pruritus and Associated Symptoms
Letters to the Editor 1 Epidemiology of Dandruff, Scalp Pruritus and Associated Symptoms Laurent Misery1,2, Nora Rahhali3, Antoine Duhamel4 and Charles Taieb3 1Laboratory of Skin Neurobiology, University of Brest, 2Department of Dermatology, University Hospital of Brest, FR-29609 Brest, 3Department of Public Health, Pierre Fabre Laboratories, Boulogne, and 4Ducray Laboratories, Lavaur, France. E-mail: [email protected] Accepted November 8, 2011. Dandruff, or pityriasis capitis, is the most common Table I. Scalp symptoms among subjects who reported dandruff [AQ1] symptom of seborrhoeic dermatitis (1). Dandruff is and those who did not usually defined as excessive flaking of the scalp. People Symptom All subjects, % Dandruff, % No dandruff, % p-value reporting dandruff often have seborrhoeic dermatitis, but Itching 21.49 51.06 15.62 < 0.0001 can also have other diseases, such as psoriasis or eczema. Prickling 14.91 31.91 11.51 < 0.0001 Although it is considered a very common condition, no Tightness 4.46 8.51 3.56 < 0.001 Pain 3.88 6.74 3.31 < 0.01 published epidemiological study evaluating the frequency Burning 2.35 4.26 1.97 0.02 of dandruff could be found in the literature. Scalp pruritus is also frequent and is often associated with dandruff. The aim of this study was to evaluate the prevalence of and severe in 11.3%, without any significant difference dandruff, scalp pruritus, and other associated symptoms, between these patients and those with pruritus without in the French population. dandruff. Patients with dandruff presented significantly more symptoms than those without dandruff: 49.3% (vs. -

Meeting Program SID 2019 ANNUAL MEETING
Meeting Program SID 2019 ANNUAL MEETING 2019 Annual Meeting Scientific 2019 Annual Meeting Program Chairs, Committee Committee on Education Members, and Reviewers Chairs and Committee CHAIRS Members Dan Kaplan, MD/PhD, University of Pittsburgh CHAIRS Ethan Lerner, MD/PhD, Mass General Hospital Heidi Kong, MD, National Insitutes of Health Todd Ridky, MD/PhD, University of Pennsylvania COMMITTEE MEMBERS Lloyd Miller, MD/PhD, Johns Hopkins University COMMITTEE MEMBERS Kevin Wang, MD/PhD, Stanford University My Mahoney, PhD, Thomas Jefferson University Spiro Getsios, PhD, Aspect Biosystems Alexander Marneros, MD/PhD, Harvard University Peggy Myung, MD/PhD, Yale University Robert Dellavalle, MD/PhD, University of Colorado Marjana Tomic-Canic PhD, University of Miami Amanda MacLeod, MD, Duke University Vladimir Botchkarev, MD/PhD, Boston University Cristina de Guzman Strong, PhD, Washington University-St. Louis Tissa Hata, MD, University of California, San Diego Maryam Asgari, MD, Massachusetts General Hospital Ken Tsai, MD/PhD, Moffitt Cancer Center and Paul Nghiem, MD/PhD, University of Washington Research Institute Richard Granstein, MD, Weill Cornell Medical School Sarah Millar, PhD, Mt. Sinai Medical School Matthew Vesley, MD/PhD, Yale University REVIEWERS Anna Di Nardo, MD/PhD Jennifer Gill, MD/PhD, University of Texas Southwestern Carolyn Lee, MD/PhD Jonathan Silverberg, MD/PhD ACKNOWLEDGEMENTS Bogi Andersen, MD The organizers of the 2019 SID Annual Meeting gratefully Kavita Sarin, MD/PhD acknowledge the sponsors, exhibitors, and participants whose Peter Koch, PhD Tiffany C. Scharschmidt, MD attendance has helped to make this meeting possible. Joseph Merola, MD Sakeen Kashem, MD/PhD Thomas Hultsch, MD Amanda MacLeod, MD Liang Deng, MD/PhD Ya-Chieh Hsu, PhD Crystal Aguh, MD Katherine Radek, PhD Paul Nghiem, MD/PhD Alicia Mathers, PhD Raymond Cho, MD Zelma Chiesa, MD Anna Mandinova, MD/PhD Brian Capell, MD/PhD Ryan R. -

Racial and Gender Differences in the Presentation of Pruritus at John Hopkins Health System and Compared the Results to Those Seen Nationally
medicines Article Racial and Gender Differences in the Presentation of Pruritus Katherine A. Whang 1, Raveena Khanna 1 , Jamael Thomas 1,2 , Crystal Aguh 1 and Shawn G. Kwatra 1,3,* 1 Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, MD 21231, USA; [email protected] (K.A.W.); [email protected] (R.K.); [email protected] (J.T.); [email protected] (C.A.) 2 School of Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA 3 Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21231, USA * Correspondence: [email protected]; Tel.: +1-410-955-8662 Received: 27 June 2019; Accepted: 25 September 2019; Published: 27 September 2019 Abstract: Background: Pruritus is a common disease symptom with a variety of etiologies known to reduce patient quality of life. We aimed to characterize the racial and gender differences in the presentation of pruritus for itch-related patient visits both within a single institution and nationally. Methods: Cross sectional study of patients 18 years old seen at Johns Hopkins Health System ≥ between 1/1/12 and 1/1/18. Results were compared to data from 2005–2011 from the National Ambulatory Medical Care Survey (NAMCS) and the National Health Ambulatory Medical Care Survey (NHAMCS). Results: Our findings indicate that itch patients at JHHS (n = 18,753) were more likely to be black compared to white patients (37% vs. 19%, p < 0.01) when compared to patients without itch—a trend also noted nationally based on data from NAMCS/NHAMCS (26% vs. 21%, p = 0.05). Black itch patients are also more likely to be diagnosed with prurigo nodularis (OR 2.37, p < 0.0001), lichen planus (OR 1.22, p < 0.0001), and atopic dermatitis OR 1.51, p < 0.0001). -
Copyrighted Material
1 Index Note: Page numbers in italics refer to figures, those in bold refer to tables and boxes. References are to pages within chapters, thus 58.10 is page 10 of Chapter 58. A definition 87.2 congenital ichthyoses 65.38–9 differential diagnosis 90.62 A fibres 85.1, 85.2 dermatomyositis association 88.21 discoid lupus erythematosus occupational 90.56–9 α-adrenoceptor agonists 106.8 differential diagnosis 87.5 treatment 89.41 chemical origin 130.10–12 abacavir disease course 87.5 hand eczema treatment 39.18 clinical features 90.58 drug eruptions 31.18 drug-induced 87.4 hidradenitis suppurativa management definition 90.56 HLA allele association 12.5 endocrine disorder skin signs 149.10, 92.10 differential diagnosis 90.57 hypersensitivity 119.6 149.11 keratitis–ichthyosis–deafness syndrome epidemiology 90.58 pharmacological hypersensitivity 31.10– epidemiology 87.3 treatment 65.32 investigations 90.58–9 11 familial 87.4 keratoacanthoma treatment 142.36 management 90.59 ABCA12 gene mutations 65.7 familial partial lipodystrophy neutral lipid storage disease with papular elastorrhexis differential ABCC6 gene mutations 72.27, 72.30 association 74.2 ichthyosis treatment 65.33 diagnosis 96.30 ABCC11 gene mutations 94.16 generalized 87.4 pityriasis rubra pilaris treatment 36.5, penile 111.19 abdominal wall, lymphoedema 105.20–1 genital 111.27 36.6 photodynamic therapy 22.7 ABHD5 gene mutations 65.32 HIV infection 31.12 psoriasis pomade 90.17 abrasions, sports injuries 123.16 investigations 87.5 generalized pustular 35.37 prepubertal 90.59–64 Abrikossoff -

Three Steps to Overcoming Scalp Itch an Emphasis on Accurate Diagnosis and Patient-Friendly Interventions Leads to Successful Management of This Common Complaint
Winter 2007 Vol. 3, No. 1 Editor’s Letter & Contributors. 3 More on Negotiations . 4 Professional Resolutions for 2007 . 4 Sun Protection Education. 7 Three Steps to Overcoming Scalp Itch An emphasis on accurate diagnosis and patient-friendly interventions leads to successful management of this common complaint. By Coyle S. Connolly, DO, and Gerard W. Stroup, PA-C hen a patient presents to the dermatology office complaining of scalp itch, he or she Wmay report frustration with the recurrent symptom, a history of failed at-home therapies, and concern about the appearance of associated lesions on the scalp. The clinician recognizes that several der- matoses may be associated with scalp itch in children and adults (Tables 1, 2). Successful management depends on accurate diagnosis and initiation of an effective, patient-friendly treatment regimen. Step 1.Visual Examination Evaluation of primary lesions will provide clues to the proper diagnosis. While thickened, silver, scaling plaques obviously indicate psoriasis, a diffuse, fine, white scale—with or without erythema—is a sign of seborrheic dermatitis. Scaling in tinea capitis tends to be well-demarcated and erythematous, with cen- tral clearing and fine peripheral scale, with or with- out associated hair loss. In African-American patients tinea capitis can resemble non-inflammato- ® 6 e Salary and Benefits: Negotiate from Strength c tions.” This is far below the American Academy of Physician i nyone seeking employment knows that the ideal posi- t tion may be elusive. Sometimes a candidate must set Assistants’ (AAPA) recently reported average national salary for c Aaside certain less important preferences in order to find full-time clinically practicing PAs of $84,396 (aapa.org). -

7Th World Congress for Hair Research
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector ABSTRACTS 7th World Congress for Hair Research ALOPECIA AREATA P001 P002 Hair follicle cycle and inflammatory infiltrate in alopecia areata at early stage Exomic sequencing of immune-related genes reveals novel candidate variants XT Zhang, Y Zhao, B Zhang, MZ Cai, YG Gong, T Ye and XQ Zhang The First Affiliated Hospital of associated with alopecia universalis Sun Yat-Sen University of Medical Sciences, Guangzhou, China HH Ryu1,SLee2,3, SH Paik1,SJJo1,J-SSeo2,3,4,J-IKim2,3,4 and OS Kwon1,5,6 1Department of Objective: To investigate the histopathological features including changing of hair follicle cycle and the Dermatology, Seoul National University College of Medicine, Seoul, Republic of Korea; 2Department inflammatory infiltrate of lymphocytes, eosinophils, and mast cells in alopecia areata (AA). of Biomedical Sciences, Seoul National University Graduate School, Seoul, Republic of Korea; Method: Clinical, laboratory, and pathological features of 136 patients with AA were 3Genomic Medicine Institute, Medical Research Center, Seoul National University, Seoul, Republic investigated. of Korea; 4Department of Biochemistry and Molecular Biology, Seoul National University College of Results: Follicular counts from biopsy specimens of the 136 patients showed largely decreased Medicine, Seoul, Republic of Korea; 5Laboratory of Cutaneous Aging and Hair Research, Clinical anagen/telogen and terminal-vellus ratios. ‘‘Swarm-of-bees’’ peribulbar lymphoid infiltration existed Research Institute, Seoul National University Hospital, Seoul, Republic of Korea and 6Institute of in 73.52% (100/136) of the patients, and those with it either had a shorter duration or had active hair Dermatological Science, Seoul National University College of Medicine, Seoul, Republic of Korea loss. -

The Clinical Conundrum of Pruritus Victoria Garcia-Albea, Karen Limaye
FEATURE ARTICLE The Clinical Conundrum of Pruritus Victoria Garcia-Albea, Karen Limaye ABSTRACT: Pruritus is a common complaint for derma- Pruritus is also the most common symptom in derma- tology patients. Diagnosing the cause of pruritus can tological disease and can be a symptom of several sys- be difficult and is often frustrating for patients and pro- temic diseases (Bernhard, 1994). The overall incidence viders. Even after the diagnosis is made, it can be a chal- of pruritus is unknown because there are no epidemio- lenge to manage and relieve pruritus. This article reviews logical databases for pruritus (Norman, 2003). According common and uncommon causes of pruritus and makes to a 2003 study, pruritus and xerosis are the most com- recommendations for proper and thorough evaluation mon dermatological problems encountered in nursing and management. home patients (Norman, 2003). Key words: Itch, Pruritus INTRODUCTION ETIOLOGY Pruritus (itch) is the most frequent symptom in derma- The skin is equipped with a network of afferent sensory tology (Serling, Leslie, & Maurer, 2011). Itch is the pre- and efferent autonomic nerve branches that respond to dominant symptom associated with acute and chronic various chemical mediators found in the skin (Bernhard, cutaneous disease and is a major symptom in systemic dis- 1994). It is believed that maximal itch production is ease (Elmariah & Lerner, 2011; Steinhoff, Cevikbas, Ikoma, achieved at the basal layer of the epidermis, below which & Berger, 2011). It can be a frustration for both the patient pain is perceived (Bernhard, 1994). Autonomic nerves in- and the clinician. This article will attempt to provide an nervate hair follicles, pili erector muscles, blood vessels, overview of pruritus, a method for thorough and com- eccrine, apocrine, and sebaceous glands (Bernhard, 1994). -

Pathogenesis and Treatment of Chronic Pruritus
Pathogenesis and of Chronic Treatment Pruritus • Shawn G. Kwatra Pathogenesis and Treatment of Chronic Pruritus Edited by Shawn G. Kwatra Printed Edition of the Special Issue Published in Medicines www.mdpi.com/journal/medicines Pathogenesis and Treatment of Chronic Pruritus Pathogenesis and Treatment of Chronic Pruritus Editor Shawn G. Kwatra MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade • Manchester • Tokyo • Cluj • Tianjin Editor Shawn G. Kwatra Johns Hopkins University School of Medicine USA Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Medicines (ISSN 2305-6320) (available at: https://www.mdpi.com/journal/medicines/special issues/pathogenesis chronic pruritus). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Article Number, Page Range. ISBN 978-3-03943-100-7 (Hbk) ISBN 978-3-03943-101-4 (PDF) c 2020 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND. Contents About the Editor .............................................. vii Kyle A. Williams and Shawn G. Kwatra Emerging Research in Chronic Pruritus: From Bedside to Bench and Back Again Reprinted from: Medicines 2020, 7, 24, doi:10.3390/medicines7050024 ............... -

Clinical Trials Protocol Template
Protocol Number: MAP-7189 Version 1.3; 28 December 2017 CLINICAL STUDY PROTOCOL A Randomized, Multi-Center, Double-blind, Placebo Controlled, Parallel Group Trial to Evaluate Efficacy and Safety of Mayne Pharma’s Ivermectin 0.5% Lotion Compared to Sklice® Ivermectin 0.5% Lotion in the Treatment of Head Lice Protocol Identifying Number: MAP-7189 Sponsor: Mayne Pharma, LLC 1240 Sugg Parkway, Greenville, NC 27834 USA Phone: +1 (252) 717-1382 Fax: +1 (252) 758-8522 Funded by: Mayne Pharma, LLC Protocol Version 1.3; 28 December2017 1 Protocol Number: MAP-7189 Version 1.3; 28 December 2017 Table of Contents SIGNATURE PAGE.............................................................................................................................................. 2 Table of Contents ................................................................................................................................................. 3 Protocol Amendment 2 Summary of Changes....................................................................................................... 6 Protocol Amendment 1 Summary of Changes....................................................................................................... 7 List of Abbreviations ............................................................................................................................................. 8 INVESTIGATOR SIGNATURE PAGE ................................................................................................................... 9 Synopsis ........................................................................................................................................................... -

Metronidazole, Topical 385
PART13.MIF Page 385 Friday, October 31, 2003 11:10 AM Metronidazole, topical 385 Methoxsalen. Dermatologic indications and dosage Disease Adult dose Child dose Component of Systemic photochemotherapy – Systemic photochemotherapy – photochemotherapy 0.4–0.6 mg per kg PO 1.5 hours 0.4–0.6 mg per kg PO 1.5 hours – psoriasis; Reiter before exposure to ultraviolet A before exposure to ultraviolet A syndrome; cutaneous light, either via light box, outdoor light, either via light box, outdoor T cell lymphoma sunlight, or photopheresis; topical sunlight, or photopheresis; topical (mycosis fungoides; therapy – 0.1% lotion applied therapy – 0.1% lotion applied Sézary syndrome; 30 minutes before exposure to 30 minutes before exposure to vitiligo; ultraviolet A light ultraviolet A light polymorphous light eruption; solar urticaria; chronic actinic dermatitis; morphea; linear scleroderma; graft versus host disease; lymphomatoid papulosis Component of 0.4–0.6 mg per kg PO 1.5 hours 0.4–0.6 mg per kg PO 1.5 hours photopheresis – T-cell before exposure to ultraviolet A before exposure to ultraviolet A lymphoma (mycosis light light fungoides; Sézary syndrome) M Contraindications/precautions Drug class Hypersensitivity to drug class or compo- Nitroimidazole antibiotic nent Mechanism of action References DNA disruption and inhibition of nucleic Laube S, George SA (2001) Adverse effects with acid synthesis (may not be mechanism in PUVA and UVB phototherapy. Journal of Der- skin disease treatment) matological Treatment 12(2):101–105 Lim HW, Edelson RL (1995) -
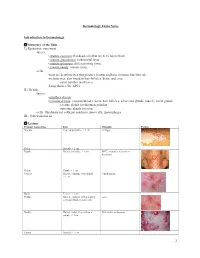
Dermatology Exam Notes Introduction to Dermatology Structure of the Skin I.) Epidermis
Dermatology Exam Notes Introduction to Dermatology Structure of the Skin I.) Epidermis: outermost -layers: • stratum corneum: flat dead cells that are 8-15 layers thick • stratum granulosum: transitional layer • stratum spinosum: differentiating tissue • stratum basale: mitotic tissue -cells: -most are keratinocytes that produce keratin and have immune function role -melanocytes: also found in hair follicles, brain, and eyes -same number in all races -Langerhans cells: APCs II.) Dermis -layers: • papillary dermis: • reticular dermis: contains blood vessels, hair follicles, sebaceous glands, muscle, sweat glands -eccrine glands for thermoregulation -apocrine glands for scent -cells: fibroblasts for collagen synthesis, mast cells, macrophages III.) Subcutaneous fat Lesions Primary lesion type Info Example Picture Macule Flat, nonpalpable, < 1 cm vitiligo Patch Macule > 1 cm Papule Raised, palpable, < 1cm BCC, psoriasis, seborrheic keratosis Plaque Papule > 1 cm Vesicle Raised, contains clear liquid, Chicken pox < 1cm Bulla Vesicle > 1cm Pustule Raised, contains inflammatory acne cells and fluid, variable size Nodule Raised, solid, deeper than a Metastatic melanoma papule, < 1cm Tumor Nodule > 1 cm 1 Wheal Firm, edematous papule or plaque that contains bound fluid, flat-topped elevations, transient Secondary lesion type Info Example Picture Scale Crust Collection of serum, blood, or pus Erosion Focal loss of epidermis that heals without scarring Ulcer Fissure Atrophy Special skin lesions Info Example Picture Excoriation Comedone Blackheads -

Download PDF (Inglês)
An Bras Dermatol. 2020;95(1):1---14 Anais Brasileiros de Dermatologia www.anaisdedermatologia.org.br CONTINUING MEDICAL EDUCATION ଝ,ଝଝ Update on parasitic dermatoses a b,∗ Alberto Eduardo Cox Cardoso , Alberto Eduardo Oiticica Cardoso , c,d e Carolina Talhari , Monica Santos a Professor Alberto Antunes University Hospital, Universidade Federal de Ciências da Saúde de Alagoas, Maceió, AL, Brazil b Department of Dermatology, Universidade Estadual de Ciências da Saúde de Alagoas, Maceió, AL, Brazil c Graduate Program of the Universidade do Estado do Amazonas, Manaus, AM, Brazil d Department of Teaching and Research, Fundac¸ão Alfredo da Matta de Dermatologia, Manaus, AM, Brazil e Department of Dermatology, Universidade do Estado do Amazonas, Tropical Dermatology Outpatient Clinic, Fundac¸ão Alfredo da Matta de Dermatologia, Manaus, AM, Brazil Received 19 February 2019; accepted 19 October 2019 Available online 31 December 2019 Abstract These are cutaneous diseases caused by insects, worms, protozoa, or coelenterates KEYWORDS which may or may not have a parasitic life. In this review the main ethological agents, clinical Drug therapy; aspects, laboratory exams, and treatments of these dermatological diseases will be studied. Larva migrans; © 2020 Sociedade Brasileira de Dermatologia. Published by Elsevier Espana,˜ S.L.U. This is an Lice infestations; open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/). Myiasis; Onchocerciasis; Scabies; Skin diseases, parasitic; Tungiasis Introduction Parasitic diseases are skin conditions caused by insects, worms, protozoa, or coelenterates that may or may not be parasitic. Given the relatively easy movement of people to ଝ different regions of the planet, knowledge of these diseases How to cite this article: Cardoso AEC, Cardoso AEO, Talhari is becoming increasingly important.