A Surgeon's Perspective on the Current Trends in Managing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
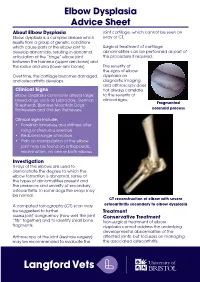
Elbow Dysplasia Advice Sheet About Elbow Dysplasia Joint Cartilage, Which Cannot Be Seen on Elbow Dysplasia Is a Complex Disease Which X-Ray Or CT
Elbow Dysplasia Advice Sheet About Elbow Dysplasia joint cartilage, which cannot be seen on Elbow dysplasia is a complex disease which x-ray or CT. results from a group of genetic conditions which cause parts of the elbow joint to Surgical treatment of cartilage develop abnormally, resulting in abnormal abnormalities can be performed as part of articulation of the “hinge” elbow joint this procedure if required. between the humerus (upper arm bone) and the radius and ulna (lower arm bones). The severity of the signs of elbow Over time, the cartilage becomes damaged, dysplasia on and osteoarthritis develops. diagnostic imaging and arthroscopy does Clinical Signs not always correlate Elbow dysplasia commonly affects large to the severity of breed dogs, such as Labradors, German clinical signs. Fragmented Shepherds, Bernese Mountain Dogs, Rottweilers and Golden Retrievers. coronoid process Clinical signs include; • Forelimb lameness and stiffness after rising or strenuous exercise • Reduced range of motion • Pain on manipulation of the elbow joint may be found on orthopaedic examination, on one or both elbows Investigation X-rays of the elbows are used to demonstrate the degree to which the elbow formation is abnormal, some of the types of abnormalities present and the presence and severity of secondary osteoarthritis. In some dogs the x-rays may be normal. CT reconstruction of elbow with severe A computed tomography (CT) scan may osteoarthritis secondary to elbow dysplasia be suggested to further Treatment assess joint congruency (how well the joint Conservative Treatment “fits” together) and to identify small bone Non-surgical treatment of elbow fragments. dysplasia cannot address the underlying developmental abnormalities of the Arthroscopy of the joint (keyhole surgery) affected joints, but focusses on managing may be recommended to evaluate the the associated osteoarthritis. -

Surgical Treatment of Canine Elbow Dysplasia (MCPD, UAP, OCD, EI)
PROCEEDINGS 33rd annual meeting of the INTERNATIONAL ELBOW WORKING GROUP September 24th 2018 WSAVA-FASAVA congress, Marina Bay Sands Congress Venue, Singapore. Welcome at the 33rd meeting of the International Elbow Working Group The International Elbow Working Group (IEWG) is an affiliate of the World Small Animal Veterinary Association (WSAVA). The board of the IEWG is therefore very grateful to the organisers of the 43rd WSAVA congress and the 9th FASAVA congress, in particular to Dr Shane Ryan, chairman of the Congress Committee, and Dr Frédéric Gaschen, chairman of the Congress Scientific Program Committee to be invited and facilitated in organizing a seminar during the pre-congress meeting of the WSAVA-2018 congress. By this invitation, the WSAVA underpins the importance of the role of the IEWG to perform the task of exchanging important information to the world veterinary community regarding the causes, prevalence, screening, therapy and prevention of one of the most important non-traumatic diseases of the locomotion system in dogs. The IEWG fulfils this task by organizing annual meetings with the help of esteemed scientist in the field of genetics, radiology, orthopedic surgery, and related fields to present the newest science and overviews on Elbow Dysplasia. Earlier this month, the IEWG had a seminar at the World Veterinary Orthopedic Congress, a joined meeting of the European Society of Veterinary Orthopedics and Traumatology (ESVOT) and the American Veterinary Orthopedic Society (VOS), with participants of European countries and the America’s. The IEWG is happy to be able to share new information with participants of the Asian and other countries coming together in Singapore. -

Sokoto Journal of Veterinary Sciences Preliminary Evaluation Of
Sokoto Journal of Veterinary Sciences, Volume 17 (Number 2). June, 2019 RESEARCH ARTICLE Sokoto Journal of Veterinary Sciences (P-ISSN 1595-093X: E-ISSN 2315-6201) http://dx.doi.org/10.4314/sokjvs.v17i2.6 Ajadi & Doyin-Dada./Sokoto Journal of Veterinary Sciences, 17(2): 45 - 53. Preliminary evaluation of prevalence of hip and elbow dysplasia in Boerboel dogs RA Ajadi *& OA Doyin-Dada Department of Veterinary Medicine and Surgery, Federal University of Agriculture, Abeokuta PMB 2240, Alabata Road, Abeokuta, Ogun State, Nigeria *Correspondence: Tel.: +2347033800326; E-mail: [email protected] Copyright: © 2019 Abstract Ajadi & Doyin-Dada. Hip dysplasia (HD) and elbow dysplasia (ED) are developmental diseases that affect This is an open-access large breed of dogs disproportionately. Despite the large size of Boerboel dogs, there article published under are no breed prevalence for HD and ED in Nigeria. This study provides preliminary the terms of the information about HD and ED prevalence in Boerboels. Twenty Boerboels of both Creative Commons sexes were evaluated. Ventrodorsal radiographs of the hip joint and flexed lateral Attribution License radiographs of elbow joint were made from each dog, using digital technology. Hip which permits grading was done using the Fédération Cynologique Internationale system (World unrestricted use, Canine Organization), assigning grades ranging from A - E. Elbow radiographs were distribution, and graded based on the International Elbow Working Group criteria, and scores ranging reproduction in any from 0-3 were assigned. Prevalence of HD and ED were expressed as percentages. medium, provided the original author and Age and sex difference were compared using a chi square test. -

Soft Tissue Calcification and Ossification
Soft Tissue Calcification and Ossification Soft-tissue Calcification Metastatic Calcification =deposit of calcium salts in previously normal tissue (1) as a result of elevation of Ca x P product above 60-70 (2) with normal Ca x P product after renal transplant Location:lung (alveolar septa, bronchial wall, vessel wall), kidney, gastric mucosa, heart, peripheral vessels Cause: (a)Skeletal deossification 1.1° HPT 2.Ectopic HPT production (lung / kidney tumor) 3.Renal osteodystrophy + 2° HPT 4.Hypoparathyroidism (b)Massive bone destruction 1.Widespread bone metastases 2.Plasma cell myeloma 3.Leukemia Dystrophic Calcification (c)Increased intestinal absorption =in presence of normal serum Ca + P levels secondary to local electrolyte / enzyme alterations in areas of tissue injury 1.Hypervitaminosis D Cause: 2.Milk-alkali syndrome (a)Metabolic disorder without hypercalcemia 3.Excess ingestion / IV administration of calcium salts 1.Renal osteodystrophy with 2° HPT 4.Prolonged immobilization 2.Hypoparathyroidism 5.Sarcoidosis 3.Pseudohypoparathyroidism (d)Idiopathic hypercalcemia 4.Pseudopseudohypoparathyroidism 5.Gout 6.Pseudogout = chondrocalcinosis 7.Ochronosis = alkaptonuria 8.Diabetes mellitus (b) Connective tissue disorder 1.Scleroderma 2.Dermatomyositis 3.Systemic lupus erythematosus (c)Trauma 1.Neuropathic calcifications 2.Frostbite 3.Myositis ossificans progressiva 4.Calcific tendinitis / bursitis (d)Infestation 1.Cysticercosis Generalized Calcinosis 2.Dracunculosis (guinea worm) (a)Collagen vascular disorders 3.Loiasis 1.Scleroderma -

Elbow Dysplasia in Dogs
Elbow dysplasia in dogs Revised by Gary Clayton Jones BVetMed DSAO DVR FRCVS with additional graphics by Jonathan Clayton Jones The British Veterinary Association and the Kennel Club — working together for excellence in canine health Elbow dysplasia has been identified as a significant problem in many breeds. Importantly, the condition appears to be increasing worldwide. It begins in puppyhood, and can affect the dog for the rest of its life The ‘flexed mediolateral’ view is a side-on view of the elbow. This view allows examination of the secondary changes in ED which occur in the shaded areas. Note how some of the shaded areas here are overlaid by other structures, which makes them difficult to examine eterinary surgeons have been Once the dog reaches skeletal maturity disease in that they have primary lesions aware for many years of a number the primary lesions may stabilise. However, or osteoarthritis in their elbows but do not of conditions that begin in puppies once abnormal development has started appear obviously lame. Some dogs will be and cause lameness. Hip dysplasia with a primary lesion, further secondary symmetrically lame in each foreleg, which Vwas the first such disease to be widely changes follow, in particular, abnormal wear can be very difficult to see. Fortunately, these recognised and a scheme for its assessment of the joint surfaces and osteoarthritis subclinical dogs can often be identified by and control is well established in the UK. (sometimes termed arthrosis, or degenerative taking radiographs (X-ray images) of their Elbow dysplasia (ED) is a significant problem joint disease — DJD). -

Proceedings 2012
PROCEEDINGS 27th annual meeting of the INTERNATIONAL ELBOW WORKING GROUP April 11th 2012 ICC, hall 7b Birmingham, UK The International Elbow Working Group acknowledges the financial support by HILL’S PET NUTRITION 27th annual meeting IEWG, Birmingham UK, April 11th 2012, p 2 WELCOME ADDRESS Birmingham, April 2012 Dear IEWG-congress participant, The International Elbow Working Group (IEWG) has been founded by a group of veterinarians and dog breeders in Davis, CA, U.S.A. in 1989 with the aim to increase the knowledge on and awareness of elbow disease in dogs, and to support all stakeholders in disseminating new knowledge in this field. The interest in hereditary aspects of elbow dysplasia (ED) has been increasing ever since both among breeders and veterinary surgeons and radiologists alike. ED has been recognized as a cause of lameness in a large variety of dog breeds with an incidence as high as 50% of the screened dogs. Published data on prevalence vary depending on breed and country. These discrepancies can be a matter of screening bias (e.g. not including dogs suffering from the disease and treated at a young age, or not submitting films of affected dogs for official screening), of improvement gained by the implementation of breeding restrictions for affected dogs, or may be caused by different screening or grading modes. The latter emphasizes the necessity of uniform grading, preferably by the protocol introduced and refined by IEWG and in use in many countries for many years. Some research groups are in a process of performing molecular genetic research in the field of canine elbow dysplasia, to use the results in the future for screening of potential breeding stock. -

University of Bradford Ethesis
University of Bradford eThesis This thesis is hosted in Bradford Scholars – The University of Bradford Open Access repository. Visit the repository for full metadata or to contact the repository team © University of Bradford. This work is licenced for reuse under a Creative Commons Licence. THE IDENTIFICATION OF BOVINE TUBERCULOSIS IN ZOOARCHAEOLOGICAL ASSEMBLAGES VOLUME 1 (1 OF 2) J. E. WOODING PhD UNIVERSITY OF BRADFORD 2010 THE IDENTIFICATION OF BOVINE TUBERCULOSIS IN ZOOARCHAEOLOGICAL ASSEMBLAGES Working towards differential diagnostic criteria Volume 1 (1 of 2) Jeanette Eve WOODING Submitted for the degree of Doctor of Philosophy School of Life Sciences Division of Archaeological, Geographical and Environmental Sciences University of Bradford 2010 Jeanette Eve WOODING The identification of bovine tuberculosis in zooarchaeological assemblages. Working towards differential diagnostic criteria. Keywords: Palaeopathology, zooarchaeology, human osteoarchaeology, zoonosis, Iron Age, Viking Age, Iceland, Orkney, England ABSTRACT The study of human palaeopathology has developed considerably in the last three decades resulting in a structured and standardised framework of practice, based upon skeletal lesion patterning and differential diagnosis. By comparison, disarticulated zooarchaeological assemblages have precluded the observation of lesion distributions, resulting in a dearth of information regarding differential diagnosis and a lack of standard palaeopathological recording methods. Therefore, zoopalaeopathology has been restricted to the analysis of localised pathologies and ‘interesting specimens’. Under present circumstances, researchers can draw little confidence that the routine recording of palaeopathological lesions, their description or differential diagnosis will ever form a standard part of zooarchaeological analysis. This has impeded the understanding of animal disease in past society and, in particular, has restricted the study of systemic disease. -

Genetic and Phenotypic Analysis of Elbow Dysplasia in Four Large Swedish Dog Breeds – an Evaluation of the Screening Programme and Clinical Symptoms
Genetic and phenotypic analysis of elbow dysplasia in four large Swedish dog breeds – an evaluation of the screening programme and clinical symptoms Genetisk och fenotypisk analys av armbågsdysplasi hos fyra storvuxna svenska hundraser – en evaluering av hälsoprogrammet och kliniska besvär Anna Medved Master Thesis • 30 hp Swedish University of Agricultural Sciences, SLU Department of Animal Breeding and Genetics Agriculture Programme – Animal Science Uppsala 2020 Genetic and phenotypic analysis of elbow dysplasia in four large Swedish dog breeds – an evaluation of the screening programme and clinical symptoms Genetisk och fenotypisk analys av armbågsdysplasi hos fyra storvuxna svenska hundraser – en evaluering av hälsoprogrammet och kliniska besvär Anna Medved Supervisor: Katja Nilsson, Swedish University of Agricultural Sciences, Department of Animal Breeding and Genetics Assistant supervisor: Sofia Malm, Geneticist at the Swedish Kennel Club Assistant supervisor: Nils Lundeheim, Swedish University of Agricultural Sciences, Department of Animal Breeding and Genetics Examiner: Erling Strandberg, Swedish University of Agricultural Sciences, Department of Animal Breeding and Genetics Credits: 30 hp Level: A2E Course title: Independent Project in Animal Science Course code: EX0872 Programme/education: Agriculture Programme – Animal Science Course coordinating dept: Place of publication: Uppsala Year of publication: 2020 Cover picture: Svenska Kennelklubben Keywords: elbow dysplasia, screening, fragmented coronoid process, osteochondritis dissecans, ununited anconeal process, elbow incongruity Swedish University of Agricultural Sciences Faculty of Veterinary Medicine and Animal Science (VH) Department of Animal Breeding and Genetics Archiving and publishing Approved students’ theses at SLU are published electronically. As a student, you have the copyright to your own work and need to approve the electronic publishing. When you have approved, metadata and full text of your thesis will be visible and searchable online. -

Australian Cattle Dog Health Testing
AUSTRALIAN CATTLE DOG HEALTH TESTING “BREEDER OF HEART” REQUIREMENTS All purebred or mixed breed dogs can have genetic and other health issues. Testing the sire and dam before breeding is a way to decrease the frequency of or prevent disorders. Your breeder should be willing and able to discuss health and genetic issues found in your chosen breed. Your breeder should also be willing to show you the proof of testing. They should be able to verify which dogs the tests relate to through means such as microchips or tattoos that correspond to the test results. They should be willing to give you copies of tests related to your new dog. Look at what the actual test results say and do your research at sites like http://www.offa.org (Orthopedic Foundation for Animals) and http://www.acdhew.org (Australian Cattle Dog Health, Education and Welfare) to understand what they mean. Even if your breeder does all suggested testing, health challenges may still arise as these are living creatures, not machines. However, as a result of testing the parents and careful breeding based on testing, the likelihood of getting a healthy puppy is higher. Testing should be done on the sire and dam before breeding as described below. Some tests can also be performed on puppies, as noted. On DNA tests, the point is to avoid producing “affected” dogs. “Carriers” of disorders can make perfectly fine pets but should only be bred to “clear” dogs to avoid producing more “affected” dogs. Sometimes the breeder has done generations of tests to produce “obligate” clear dogs so the puppies themselves are no longer tested for certain disorders. -

Osteochondrosis – Primary Epiphyseal (Articular/Subchondral) Lesion Can Heal Or Can Progress
60 120 180 1 distal humeral condyles 2 medial epicondyle 3 proximal radial epiphysis 4 anconeal process Lab Ret study N=1018 . Normal . Affected . Total 688 (67.6%) . Total 330 (32.4%) . Male 230 (62.2%) . Male 140 (37.8%) . Female 458 (70.7%) . Female 190 (29.3%) Affected dogs N=330 1affected site - 250 (75.7%) 2 affected sites - 68 (20.6%) 3 affected sites - 12 (3.6%) immature skeletal diseases denis novak technique for skeletal radiography tissue < 12 cm “non-grid” (“table-top”) technique “high detail” system radiation safety diagnosis X – rays examination Ultrasound CT bilateral lesions - clinical signs ? unilateral present > one type of lesion 2ry arthrosis Common Osteochondrosis – primary epiphyseal (articular/subchondral) lesion can heal or can progress Osteochondritis dissecans – free articular fragment will progress Arthrosis Osteochondrosis talus / tarsus Lumbosacral OCD Lumbosacral OCD Inflammatory diseases Panosteitis – non infectious Hypertrophic osteodystrophy (HOD) – perhaps infectious Osteomyelitis - infectious Panosteitis New medullary bone Polyostotic Multiple lesions in one bone Symmetrical or nonsymmetrical Sclerotic pattern B I L A T E R A L periosteal new bone forms with chronicity Cross sections of a tibia different locations Hypertrophic osteodystrophy (HOD) Dogs are systemically ill, febrile, anorectic, reluctant to walk most will recover Radiographic changes of HOD . Polyostotic . Metaphyseal . Symmetrical . Changes of lesion Early Mid Late lytic “plates” in acute case HOD - 4 m ret – lesions are present -

Hyperostosis Corticalis Infantalis (Caffey's Disease)* J
754 S.A. TYDSKRIF VIR GENEESKU DE 14 Junie 1969 HYPEROSTOSIS CORTICALIS INFANTALIS (CAFFEY'S DISEASE)* J. M. WAGNER, M.B., B.CH., M.R.e.p., Senior Paediatrician, Edenvale Hospital, AND A. SoLOMON, M.B., B.CH., DIP.MED., D.M.R.(D.), Radiologist, Pneumoconiosis Research Unit, CSIR, Baragwanath Hospital, Johannesburg Infantile cortical hyperostosis is a disorder affecting the skeleton and some of its contiguous fascias and muscles. It is suggested that infantile cortical hyperostosis is a pre natal collagen disease.' The early stage is of acute inflammation and loss of the periosteal and subperiosteal definition. There is a fibrous and osteoblastic reaction and overlying tissue including muscle is involved. No bacteria are seen. Later, there is subperiosteal new lamellar bone formation. The peri osteum is thickened and hyperplastic and the overlying soft tissues are oedematous and sections show round-cell infiltration. The subacute phase re-establishes periosteum as an entity. The later remodelling stage removes the extraperipheral bone from within, resulting in dilatation of the medullary cavity. There is evidence to suggest that infantile cortical hyper ostosis had been recognized in 1930.' Caffey and Silver man first described the disease in 1946." Smyth et al.' also recorded cases in 1946. Altogether 102 cases have been reported, Sidbury and Sidbury' contributing 69 reports and Holrnan' describing 33 cases. Infantile cortical hyper ostosis has been described in Negroes but seems rare in the South African Bantu. CASE REPORT The patient, a 3-year-old Bantu male, had a normal birth weight. Two siblings were in good health. The mother stated that the child's jaw was swollen and that he had Fig. -

Musculoskeletal Radiology
MUSCULOSKELETAL RADIOLOGY Developed by The Education Committee of the American Society of Musculoskeletal Radiology 1997-1998 Charles S. Resnik, M.D. (Co-chair) Arthur A. De Smet, M.D. (Co-chair) Felix S. Chew, M.D., Ed.M. Mary Kathol, M.D. Mark Kransdorf, M.D., Lynne S. Steinbach, M.D. INTRODUCTION The following curriculum guide comprises a list of subjects which are important to a thorough understanding of disorders that affect the musculoskeletal system. It does not include every musculoskeletal condition, yet it is comprehensive enough to fulfill three basic requirements: 1.to provide practicing radiologists with the fundamentals needed to be valuable consultants to orthopedic surgeons, rheumatologists, and other referring physicians, 2.to provide radiology residency program directors with a guide to subjects that should be covered in a four year teaching curriculum, and 3.to serve as a “study guide” for diagnostic radiology residents. To that end, much of the material has been divided into “basic” and “advanced” categories. Basic material includes fundamental information that radiology residents should be able to learn, while advanced material includes information that musculoskeletal radiologists might expect to master. It is acknowledged that this division is somewhat arbitrary. It is the authors’ hope that each user of this guide will gain an appreciation for the information that is needed for the successful practice of musculoskeletal radiology. I. Aspects of Basic Science Related to Bone A. Histogenesis of developing bone 1. Intramembranous ossification 2. Endochondral ossification 3. Remodeling B. Bone anatomy 1. Cellular constituents a. Osteoblasts b. Osteoclasts 2. Non cellular constituents a.