KHON KAEN Udon Thani 145 X 210 Mm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ancient Genomes Document Multiple Waves of Migration in Southeast
Ancient genomes document multiple waves of migration in Southeast Asian prehistory Mark Lipson, Olivia Cheronet, Swapan Mallick, Nadin Rohland, Marc Oxenham, Michael Pietrusewsky, Thomas Oliver Pryce, Anna Willis, Hirofumi Matsumura, Hallie Buckley, et al. To cite this version: Mark Lipson, Olivia Cheronet, Swapan Mallick, Nadin Rohland, Marc Oxenham, et al.. Ancient genomes document multiple waves of migration in Southeast Asian prehistory. Science, American Association for the Advancement of Science, 2018, 361 (6397), pp.92 - 95. 10.1126/science.aat3188. cea-01870144 HAL Id: cea-01870144 https://hal-cea.archives-ouvertes.fr/cea-01870144 Submitted on 19 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. RESEARCH HUMAN GENOMICS wide data using in-solution enrichment, yielding sequences from 18 individuals (Table 1 and table S1) (19). Because of poor preservation conditions in tropical environments, we observed both a low Ancient genomes document multiple rate of conversion of screened samples to work- ing data and also limited depth of coverage per waves of migration in Southeast sample, and thus we created multiple libraries per individual (102 in total in our final dataset). Asian prehistory We initially analyzed the data by performing principal component analysis (PCA) using two different sets of present-day populations (19). -

Section II: Periodic Report on the State of Conservation of the Ban Chiang
Thailand National Periodic Report Section II State of Conservation of Specific World Heritage Properties Section II: State of Conservation of Specific World Heritage Properties II.1 Introduction a. State Party Thailand b. Name of World Heritage property Ban Chiang Archaeological Site c. Geographical coordinates to the nearest second North-west corner: Latitude 17º 24’ 18” N South-east corner: Longitude 103º 14’ 42” E d. Date of inscription on the World Heritage List December 1992 e. Organization or entity responsible for the preparation of the report Organization (s) / entity (ies): Ban Chiang National Museum, Fine Arts Department - Person (s) responsible: Head of Ban Chiang National Museum, Address: Ban Chiang National Museum, City and Post Code: Nhonghan District, Udonthanee Province 41320 Telephone: 66-42-208340 Fax: 66-42-208340 Email: - f. Date of Report February 2003 g. Signature on behalf of State Party ……………………………………… ( ) Director General, the Fine Arts Department 1 II.2 Statement of significance The Ban Chiang Archaeological Site was granted World Heritage status by the World Heritage Committee following the criteria (iii), which is “to bear a unique or at least exceptional testimony to a cultural tradition or to a civilization which is living or which has disappeared ”. The site is an evidence of prehistoric settlement and culture while the artifacts found show a prosperous ancient civilization with advanced technology which had evolved for 5,000 years, such as rice farming, production of bronze and metal tools, and the production of pottery which had its own distinctive characteristics. The prosperity of the Ban Chiang culture also spread to more than a hundred archaeological sites in the Northeast of Thailand. -

(Unofficial Translation) Order of the Centre for the Administration of the Situation Due to the Outbreak of the Communicable Disease Coronavirus 2019 (COVID-19) No
(Unofficial Translation) Order of the Centre for the Administration of the Situation due to the Outbreak of the Communicable Disease Coronavirus 2019 (COVID-19) No. 1/2564 Re : COVID-19 Zoning Areas Categorised as Maximum COVID-19 Control Zones based on Regulations Issued under Section 9 of the Emergency Decree on Public Administration in Emergency Situations B.E. 2548 (2005) ------------------------------------ Pursuant to the Declaration of an Emergency Situation in all areas of the Kingdom of Thailand as from 26 March B.E. 2563 (2020) and the subsequent 8th extension of the duration of the enforcement of the Declaration of an Emergency Situation until 15 January B.E. 2564 (2021); In order to efficiently manage and prepare the prevention of a new wave of outbreak of the communicable disease Coronavirus 2019 in accordance with guidelines for the COVID-19 zoning based on Regulations issued under Section 9 of the Emergency Decree on Public Administration in Emergency Situations B.E. 2548 (2005), by virtue of Clause 4 (2) of the Order of the Prime Minister No. 4/2563 on the Appointment of Supervisors, Chief Officials and Competent Officials Responsible for Remedying the Emergency Situation, issued on 25 March B.E. 2563 (2020), and its amendments, the Prime Minister, in the capacity of the Director of the Centre for COVID-19 Situation Administration, with the advice of the Emergency Operation Center for Medical and Public Health Issues and the Centre for COVID-19 Situation Administration of the Ministry of Interior, hereby orders Chief Officials responsible for remedying the emergency situation and competent officials to carry out functions in accordance with the measures under the Regulations, for the COVID-19 zoning areas categorised as maximum control zones according to the list of Provinces attached to this Order. -

The Mineral Industry of Thailand in 2008
2008 Minerals Yearbook THAILAND U.S. Department of the Interior August 2010 U.S. Geological Survey THE MINERAL INDUS T RY OF THAILAND By Lin Shi In 2008, Thailand was one of the world’s leading producers by 46% to 17,811 t from 32,921 t in 2007. Production of iron of cement, feldspar, gypsum, and tin. The country’s mineral ore and Fe content (pig iron and semimanufactured products) production encompassed metals, industrial minerals, and each increased by about 10% to 1,709,750 t and 855,000 t, mineral fuels (table 1; Carlin, 2009; Crangle, 2009; Potter, 2009; respectively; manganese output increased by more than 10 times van Oss, 2009). to 52,700 t from 4,550 t in 2007, and tungsten output increased by 52% to 778 t from 512 t in 2007 (table 1). Minerals in the National Economy Among the industrial minerals, production of sand, silica, and glass decreased by 41%; that of marble, dimension stone, and Thailand’s gross domestic product (GDP) in 2008 was fragment, by 22%; and pyrophyllite, by 74%. Production of ball valued at $274 billion, and the annual GDP growth rate was clay increased by 166% to 1,499,993 t from 563,353 t in 2007; 2.6%. The growth rate of the mining sector’s portion of the calcite and dolomite increased by 22% each; crude petroleum GDP increased by 0.6% compared with that of 2007, and that oil increased by 9% to 53,151 barrels (bbl) from 48,745 bbl in of the manufacturing sector increased by 3.9%. -
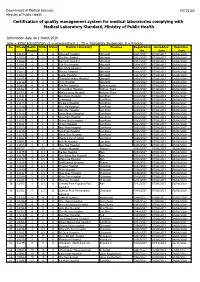
Certification of Quality Management System for Medical Laboratories Complying with Medical Laboratory Standard, Ministry of Public Health
Department of Medical Sciences F0715102 Ministry of Public Health Certification of quality management system for medical laboratories complying with Medical Laboratory Standard, Ministry of Public Health Information date on 1 March 2019 new = initial accreditation, r1 = reassessment 1 , TS = Temporary Suspension, P = Process No. HCode Health RMSc Status Medical Laboratory Province Registration Accredited Expiration Region No. Date Date 1 10673 2 2 r1 Uttaradit Hospital Uttaradit 0001/2557 07/08/2017 06/08/2020 2 11159 2 2 r1 Tha Pla Hospital Uttaradit 0002/2557 07/08/2017 06/08/2020 3 11160 2 2 r1 Nam Pat Hospital Uttaradit 0003/2557 07/08/2017 06/08/2020 4 11161 2 2 r1 Fak Tha Hospital Uttaradit 0004/2557 07/08/2017 06/08/2020 5 11162 2 2 r1 Ban Khok Hospital Uttaradit 0005/2557 07/08/2017 06/08/2020 6 11163 2 2 r1 Phichai Hospital Uttaradit 0006/2557 07/08/2017 06/08/2020 7 11164 2 2 r1 Laplae Hospital Uttaradit 0007/2557 07/08/2017 06/08/2020 8 11165 2 2 r1 ThongSaenKhan Hospital Uttaradit 0008/2557 07/08/2017 06/08/2020 9 11158 2 2 r1 Tron Hospital Uttaradit 0009/2557 07/08/2017 06/08/2020 10 10863 4 4 r1 Pak Phli Hospital Nakhonnayok 0010/2557 07/08/2017 06/08/2020 11 10762 4 4 r1 Thanyaburi Hospital Pathum Thani 0011/2557 07/08/2017 06/08/2020 12 10761 4 4 r1 Klong Luang Hospital Pathum Thani 0012/2557 07/08/2017 06/08/2020 13 11141 1 1 P Ban Hong Hospital LamPhun 0014/2557 07/08/2014 06/08/2017 14 11142 1 1 P Li Hospital LamPhun 0015/2557 07/08/2014 06/08/2017 15 11144 1 1 P Pa Sang Hospital LamPhun 0016/2557 07/08/2014 06/08/2017 -

Siam Makro Public Company Limited and Its Subsidiaries
VISION To Be Number One Food Solution Provider For Professional Customers MISSION MultiM - formats Award Know theK differences ResponsibleR OfferO the best Expansion Team achievement Know and For the society Value & solution and invest in people respect the local and the world to customers differences CONTENT Financial Highlights 002 Message from the Chairman and Group Chief Executive Officer - Siam Makro 004 Corporate Governance Committee's Report 006 Nomination and Remuneration Committee's Report 008 Moving Far and Growing Strong 010 Growing Together in Hearts and in Minds 018 The Creative Journey to Sustainability 032 Human Resources and Organizational Development 044 Environmental, Occupational Health and Safety Management 053 2018 Key Milestones 057 Market Overview and Industry Outlook 062 Future Projects 065 Shareholding and Management Structure 068 Board of Directors and Executives 089 Corporate Risk Factors and Risk Management 108 Corporate Governance 113 Corporate Information 133 Audit Committee’s Report 144 Management’s Discussion and Analysis of Financial Position and 146 Operating Results Related Party Transactions 151 Report of the Board of Directors’ Responsibilities for Financial Statements 158 Financial Statements 159 Locations 243 002 Annual Report 2018 192,930 186,574 Total revenues 172,790 3.3% Over 2017 2016 2017 20182017 6,178 5,942 Net profit 5,413 Down3.8% 2017 2016 2017 20182560 62,065 58,976 Total assets 52,859 5.2%Over 2017 2016 25602017 2018 Siam Makro Public Company Limited 003 Financial Highlights As at 31 -

Nong Khai Nong Khai Nong Khai 3 Mekong River
Nong Khai Nong Khai Nong Khai 3 Mekong River 4 Nong Khai 4 CONTENTS HOW TO GET THERE 7 ATTRACTIONS 9 Amphoe Mueang Nong khai 9 Amphoe Tha Bo 16 Amphoe Si Chiang Mai 17 Amphoe Sangkhom 18 Amphoe Phon Phisai 22 Amphoe Rattanawapi 23 EVENTS AND FESTIVALS 25 LOCAL PRODUCTS 25 SOUVENIR SHOPS 26 SUGGESTED ITINERARY 26 FACILITIES 27 Accommodations 27 Restaurants 30 USEFUL CALLS 31 Nong Khai 5 5 Wat Aranyabanpot Nong Khai 6 Thai Term Glossary a rebellion. King Rama III appointed Chao Phraya Amphoe: District Ratchathewi to lead an army to attack Vientiane. Ban: Village The army won with the important forces Hat: Beach supported by Thao Suwothanma (Bunma), Khuean: Dam the ruler of Yasothon, and Phraya Chiangsa. Maenam: River The king, therefore, promoted Thao Suwo to Mueang: Town or City be the ruler of a large town to be established Phrathat: Pagoda, Stupa on the right bank of the Mekong River. The Prang: Corn-shaped tower or sanctuary location of Ban Phai was chosen for the town SAO: Subdistrict Administrative Organization called Nong Khai, which was named after a very Soi: Alley large pond to the west. Song Thaeo: Pick-up trucks but with a roof Nong Khai is 615 kilometres from Bangkok, over the back covering an area of around 7,332 square Talat: Market kilometres. This province has the longest Tambon: Subdistrict distance along the Mekong River; measuring Tham: Cave 320 kilometres. The area is suitable for Tuk-Tuks: Three-wheeled motorized taxis agriculture and freshwater fishery. It is also Ubosot or Bot: Ordination hall in a temple a major tourist attraction where visitors can Wihan: Image hall in a temple easily cross the border into Laos. -

Changing Paradigms in Southeast Asian Archaeology
CHANGING PARADIGMS IN SOUTHEAST ASIAN ARCHAEOLOGY Joyce C. White Institute for Southeast Asian Archaeology and University of Pennsylvania Museum ABSTRACT (e.g., Tha Kae, Ban Mai Chaimongkol, Non Pa Wai, and In order for Southeast Asian archaeologists to effectively many other sites in central Thailand; but see White and engage with global archaeological discussions of the 21st Hamilton [in press] for progress on Ban Chiang). century, adoption of new paradigms is advocated. The But what I want to focus on here is our paradigmatic prevalent mid-twentieth century paradigm’s reliance on frameworks. Paradigms — that set of assumptions, con- essentialized frameworks and directional macro-views cepts, values, and practices that underlie an intellectual dis- should be replaced with a forward-facing, “emergent” cipline at particular points in time — matter. They matter paradigm and an emphasis on community-scale analyses partly because if we are parroting an out-of-date archaeo- in alignment with current trends in archaeological theory. logical agenda, we will miss out on three important things An example contrasting the early i&i pottery with early crucial for the vitality of the discipline of Southeast Asian copper-base metallurgy in Thailand illustrates how this archaeology in the long term. First is institutional support new perspective could approach prehistoric data. in terms of jobs. Second is resources. In both cases, appli- cants for jobs and grants need to be in tune with scholarly trends. Third, what interests me most in this paper, is our place in global archaeological discussions. Participating in INTRODUCTION global archaeological conversations, being a player in tune with the currents of the time, tends to assist in gaining in- When scholars reach the point in their careers that they are 1 stitutional support and resources. -

Contracted Garage
Contracted Garage No Branch Province District Garage Name Truck Contact Number Address 035-615-990, 089- 140/2 Rama 3 Road, Bang Kho Laem Sub-district, Bang Kho Laem District, 1 Headquarters Ang Thong Mueang P Auto Image Co., Ltd. 921-2400 Bangkok, 10120 188 Soi 54 Yaek 4 Rama 2 Road, Samae Dam Sub-district, Bang Khun Thian 2 Headquarters Ang Thong Mueang Thawee Car Care Center Co., Ltd. 035-613-545 District, Bangkok, 10150 02-522-6166-8, 086- 3 Headquarters Bangkok Bang Khen Sathitpon Aotobody Co., Ltd. 102/8 Thung Khru Sub-district, Thung Khru District, Bangkok, 10140 359-7466 02-291-1544, 081- 4 Headquarters Bangkok Bang Kho Laem Au Supphalert Co., Ltd. 375 Phet kasem Road, Tha Phra Sub-district, Bangkok Yai District, Bangkok, 10600 359-2087 02-415-1577, 081- 109/26 Moo 6 Nawamin 74 Road Khlong Kum Sub-district Bueng Kum district 5 Headquarters Bangkok Bang Khun Thian Ch.thanabodyauto Co., Ltd. 428-5084 Bangkok, 10230 02-897-1123-8, 081- 307/201 Charansanitwong Road, Bang Khun Si Sub-district, Bangkok Noi District, 6 Headquarters Bangkok Bang Khun Thian Saharungroj Service (2545) Co., Ltd. 624-5461 Bangkok, 10700 02-896-2992-3, 02- 4/431-3 Moo 1, Soi Sakae Ngam 25, Rama 2 Road, Samae Dam 7 Headquarters Bangkok Bang Khun Thian Auychai Garage Co., Ltd. 451-3715 Sub-district, Bang Khun Thien District, Bangkok, 10150 02-451-6334, 8 Headquarters Bangkok Bang Khun Thian Car Circle and Service Co., Ltd. 495 Hathairat Road, Bang, Khlong Sam Wa District, Bangkok, 10510 02-451-6927-28 02-911-5001-3, 02- 9 Headquarters Bangkok Bang Sue Au Namchai TaoPoon Co., Ltd. -

Meaning Relationship Between the Original Ban Chiang Pottery Pattern Stamps and Modern Auspicious Patterns
Kasetsart Journal of Social Sciences 42 (2021) 439–446 Kasetsart Journal of Social Sciences journal homepage: http://kjss.kasetsart.org Meaning relationship between the original Ban Chiang pottery pattern stamps and modern auspicious patterns Kanittha Ruangwannasaka,*, Sutthinee Sukkulb,†, Atchariya Suriyac, Krissada Dupandungd a Faculty of Humanities and Social science, Udon Thani Rajabhat University, Mueang, Udon Thani 41000, Thailand b Faculty of Architecture, King Mongkut’s Institute of Technology Ladkrabang, Lat Krabang, Bangkok 10520, Thailand c Faculty of Technology, Udon Thani Rajabhat University, Mueang, Udon Thani 41000, Thailand d Faculty of Art and Industrial Design, Rajamangala University of Technology Isan, Mueang, Nakhon Ratchasima 30000, Thailand Article Info Abstract Article history: The study investigates the relationship between the original Ban Chiang patterns and Received 19 March 2020 Revised 27 April 2020 modern auspicious patterns in terms of meaning. The qualitative method was used to Accepted 5 May 2020 collect and analyze data. The sample or information source of Ban Chiang clay rollers Available online 30 April 2021 and Ban Chiang pottery pattern stamps includes a curator and a storekeeper of the Ban Chiang National Museum and a local person well-informed in Ban Chiang. Results Keywords: revealed that the Ban Chiang patterns are similar to modern auspicious patterns mainly Ban Chiang clay roller, in the use of animals; nature; fruits, trees, and flowers; objects, utensils, or artificial Ban Chiang pottery pattern stamp, designs; and belief as symbols. modern auspicious pattern, relationship © 2021 Kasetsart University. Introduction The researchers conducted interviews with sellers of goods and souvenirs to identify issues related to tourism and The Ministry of Tourism and Sports (2015) revealed that maximize the many tourist sites in Udon Thani and yearly one of the factors influencing tourism in Thailand that increasing number of tourists. -

Aw-Poster-Pongsak Pirom-0629
Poster #0629 HEPATITIS B VIRUS DNA LEVEL CHANGES IN HBeAg+ PREGNANT WOMEN RECEIVING TDF FOR PREVENTION OF MOTHER-TO-CHILD TRANSMISSION IRD-CMU PHPT CROIConference on Retroviruses Nicole Ngo-Giang-Huong1, Nicolas Salvadori2, Woottichai Khamduang2, Tim R. Cressey2, Linda J. Harrison3, Luc Decker1, Camlin Tierney3, Jullapong Achalapong4, and Opportunistic Infections Trudy V. Murphy5, Noele Nelson5, George K. Siberry6, Raymond T. Chung7, Stanislas Pol8, Gonzague Jourdain1, for the iTAP study group 1IRD, Chiang Mai, Thailand, 2Chiang Mai University, Chiang Mai, Thailand, 3Harvard University, Boston, MA, USA, 4Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand, 5CDC, Atlanta, GA, USA, 6USAID, Arlington, VA, USA, 7Massachusetts General Hospital, Boston, MA, USA, 8Cochin Hospital, Paris, France Background HBV DNA load measurements • 12% (19 of 161) did not achieve 5.3 log10 IU/ml at delivery; References • Population: all women assigned to the TDF arm + a randomly the median (range) HBV DNA for these women was 8.3 • High hepatitis B virus (HBV) DNA levels and positive hepatitis (7.1 to 9.1) log IU/mL at baseline, 7.4 (4.7 to 8.6) at • Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on selected subset of 50 women assigned to the placebo arm 10 B e antigen (HBeAg-an indicator of rapid viral replication and 32-weeks, 7.0 (3.9 to 8.5) at 36 weeks and 7.8 (5.3 to 8.9) the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98. • European Association for the Study of the Liver. Electronic address eee, high level of HBV DNA) are the main markers of risk for • Timing: at baseline (28 weeks gestation), at Weeks 32 and at delivery. -

Gas Stations
Gas Stations Chuchawal Royal Haskoning was responsible for the construction Country: management and site supervision for 3 Q8 Gas filling stations; Kaeng Koi Thailand District in Saraburi Province, Bangyai District in Nonthaburi Province and in Chonburi. Client: Q8 The Q8 gas stations are constructed on 4-rai areas. Each station comprises a 350 m2 convenience store, 4 multi-product fuel dispensers, canopies, public Period: toilets, customers’ relaxation area with shelter, sign board and staff housing. 2002 Chuchawal Royal Haskoning 8th Flr., Asoke Towers, 219/25 Sukhumvit 21, Bangkok 10110. Tel: +66 259 1186. Fax: +66 260 0230 Internet: www.chuchawalroyalhaskoning.com Reference: T/0232 HomePro Store HomePro is a very successful company engaged in the sale of hardware, Country: furniture and home improvement products both for the retail market and for Bangkok, Thailand small contractors. Several new stores were built in 2002 and 2003. Client: Chuchawal Royal Haskoning, in cooperation with its sister company, Interior Home Product Center PCL. Architecture 103, provided project management and construction management and site supervision services for the construction of their new stores. Period: The HomePro – Rama II store comprises 3 buildings with a total retail area of 2002 10,500m2. Chuchawal Royal Haskoning 8th Flr., Asoke Towers, 219/25 Sukhumvit 21, Bangkok 10110. Tel: +66 259 1186. Fax: +66 260 0230 Internet: www.chuchawalroyalhaskoning.com Reference: T/0233 Water Resources Assessment for proposed Horticultural Farm Green and Clean Vegetables wanted to establish a new horticultural farm in Country: Muak Lek district, Saraburi. For their water resource they required a good- Saraburi, Thailand quality and reliable water supply.