WO 2015/036583 A2 19 March 2015 (19.03.2015) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Overview of the Role of Hdacs in Cancer Immunotherapy
International Journal of Molecular Sciences Review Immunoepigenetics Combination Therapies: An Overview of the Role of HDACs in Cancer Immunotherapy Debarati Banik, Sara Moufarrij and Alejandro Villagra * Department of Biochemistry and Molecular Medicine, School of Medicine and Health Sciences, The George Washington University, 800 22nd St NW, Suite 8880, Washington, DC 20052, USA; [email protected] (D.B.); [email protected] (S.M.) * Correspondence: [email protected]; Tel.: +(202)-994-9547 Received: 22 March 2019; Accepted: 28 April 2019; Published: 7 May 2019 Abstract: Long-standing efforts to identify the multifaceted roles of histone deacetylase inhibitors (HDACis) have positioned these agents as promising drug candidates in combatting cancer, autoimmune, neurodegenerative, and infectious diseases. The same has also encouraged the evaluation of multiple HDACi candidates in preclinical studies in cancer and other diseases as well as the FDA-approval towards clinical use for specific agents. In this review, we have discussed how the efficacy of immunotherapy can be leveraged by combining it with HDACis. We have also included a brief overview of the classification of HDACis as well as their various roles in physiological and pathophysiological scenarios to target key cellular processes promoting the initiation, establishment, and progression of cancer. Given the critical role of the tumor microenvironment (TME) towards the outcome of anticancer therapies, we have also discussed the effect of HDACis on different components of the TME. We then have gradually progressed into examples of specific pan-HDACis, class I HDACi, and selective HDACis that either have been incorporated into clinical trials or show promising preclinical effects for future consideration. -

Hr23b Expression Is a Potential Predictive Biomarker for HDAC Inhibitor Treatment in Mesenchymal Tumours and Is Associated with Response to Vorinostat
The Journal of Pathology: Clinical Research J Path: Clin Res April 2016; 2: 59–71 Original Article Published online 23 December 2015 in Wiley Online Library (wileyonlinelibrary.com). DOI: 10.1002/cjp2.35 HR23b expression is a potential predictive biomarker for HDAC inhibitor treatment in mesenchymal tumours and is associated with response to vorinostat Michaela Angelika Ihle,1 Sabine Merkelbach-Bruse,1 Wolfgang Hartmann,1,2 Sebastian Bauer,3 Nancy Ratner,4 Hiroshi Sonobe,5 Jun Nishio,6 Olle Larsson,7 Pierre A˚ man,8 Florence Pedeutour,9 Takahiro Taguchi,10 Eva Wardelmann,1,2 Reinhard Buettner1 and Hans-Ulrich Schildhaus1,11* 1 Institute of Pathology, University Hospital Cologne, Cologne, Germany 2 Gerhard Domagk Institute of Pathology, University Hospital M€unster, M€unster, Germany 3 Sarcoma Center, West German Cancer Center, University of Essen, Essen, Germany 4 US Department of Pediatrics, Cincinnati Children’s Hospital Medical Centre, Cincinnati, OH, USA 5 Department of Laboratory Medicine, Chugoku Central Hospital, Fukuyama, Hiroshima, Japan 6 Faculty of Medicine, Department of Orthopaedic Surgery, Fukuoka University, Fukuoka, Japan 7 Department of Oncology and Pathology, The Karolinska Institute, Stockholm, Sweden 8 Sahlgrenska Cancer Centre, University of Gothenburg, Gothenburg, Sweden 9 Faculty of Medicine, Laboratory of Genetics of Solid Tumours, Institute for Research on Cancer and Aging, Nice, France 10 Division of Human Health & Medical Science, Graduate School of Kuroshio Science, Kochi University Nankoku, Kochi, Japan 11 Institute of Pathology, University Hospital G€ottingen, G€ottingen, Germany *Correspondence to: Hans-Ulrich Schildhaus,Institute of Pathology,University Hospital G€ottingen,Robert-Koch-Strasse40,D-37075G€ottingen, Germany.e-mail: [email protected] Abstract Histone deacetylases (HDAC) are key players in epigenetic regulation of gene expression and HDAC inhibitor (HDACi) treatment seems to be a promising anticancer therapy in many human tumours, including soft tissue sar- comas. -

Histone Deacetylase 3 As a Novel Therapeutic Target in Multiple Myeloma
Leukemia (2014) 28, 680–689 & 2014 Macmillan Publishers Limited All rights reserved 0887-6924/14 www.nature.com/leu ORIGINAL ARTICLE Histone deacetylase 3 as a novel therapeutic target in multiple myeloma J Minami1,4, R Suzuki1,4, R Mazitschek2, G Gorgun1, B Ghosh2,3, D Cirstea1,YHu1, N Mimura1, H Ohguchi1, F Cottini1, J Jakubikova1, NC Munshi1, SJ Haggarty3, PG Richardson1, T Hideshima1 and KC Anderson1 Histone deacetylases (HDACs) represent novel molecular targets for the treatment of various types of cancers, including multiple myeloma (MM). Many HDAC inhibitors have already shown remarkable antitumor activities in the preclinical setting; however, their clinical utility is limited because of unfavorable toxicities associated with their broad range HDAC inhibitory effects. Isoform- selective HDAC inhibition may allow for MM cytotoxicity without attendant side effects. In this study, we demonstrated that HDAC3 knockdown and a small-molecule HDAC3 inhibitor BG45 trigger significant MM cell growth inhibition via apoptosis, evidenced by caspase and poly (ADP-ribose) polymerase cleavage. Importantly, HDAC3 inhibition downregulates phosphorylation (tyrosine 705 and serine 727) of signal transducers and activators of transcription 3 (STAT3). Neither interleukin-6 nor bone marrow stromal cells overcome this inhibitory effect of HDAC3 inhibition on phospho-STAT3 and MM cell growth. Moreover, HDAC3 inhibition also triggers hyperacetylation of STAT3, suggesting crosstalk signaling between phosphorylation and acetylation of STAT3. Importantly, inhibition of HDAC3, but not HDAC1 or 2, significantly enhances bortezomib-induced cytotoxicity. Finally, we confirm that BG45 alone and in combination with bortezomib trigger significant tumor growth inhibition in vivo in a murine xenograft model of human MM. -

Impact of the Microbial Derived Short Chain Fatty Acid Propionate on Host Susceptibility to Bacterial and Fungal Infections in Vivo
www.nature.com/scientificreports OPEN Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and Received: 01 July 2016 Accepted: 02 November 2016 fungal infections in vivo Published: 29 November 2016 Eleonora Ciarlo1,*, Tytti Heinonen1,*, Jacobus Herderschee1, Craig Fenwick2, Matteo Mombelli1, Didier Le Roy1 & Thierry Roger1 Short chain fatty acids (SCFAs) produced by intestinal microbes mediate anti-inflammatory effects, but whether they impact on antimicrobial host defenses remains largely unknown. This is of particular concern in light of the attractiveness of developing SCFA-mediated therapies and considering that SCFAs work as inhibitors of histone deacetylases which are known to interfere with host defenses. Here we show that propionate, one of the main SCFAs, dampens the response of innate immune cells to microbial stimulation, inhibiting cytokine and NO production by mouse or human monocytes/ macrophages, splenocytes, whole blood and, less efficiently, dendritic cells. In proof of concept studies, propionate neither improved nor worsened morbidity and mortality parameters in models of endotoxemia and infections induced by gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae), gram-positive bacteria (Staphylococcus aureus, Streptococcus pneumoniae) and Candida albicans. Moreover, propionate did not impair the efficacy of passive immunization and natural immunization. Therefore, propionate has no significant impact on host susceptibility to infections and the establishment of protective anti-bacterial responses. These data support the safety of propionate- based therapies, either via direct supplementation or via the diet/microbiota, to treat non-infectious inflammation-related disorders, without increasing the risk of infection. Host defenses against infection rely on innate immune cells that sense microbial derived products through pattern recognition receptors (PRRs) such as toll-like receptors (TLRs), c-type lectins, NOD-like receptors, RIG-I-like receptors and cytosolic DNA sensors. -

Valproic Acid and Breast Cancer: State of the Art in 2021
cancers Review Valproic Acid and Breast Cancer: State of the Art in 2021 Anna Wawruszak 1,* , Marta Halasa 1, Estera Okon 1, Wirginia Kukula-Koch 2 and Andrzej Stepulak 1 1 Department of Biochemistry and Molecular Biology, Medical University of Lublin, 20-093 Lublin, Poland; [email protected] (M.H.); [email protected] (E.O.); [email protected] (A.S.) 2 Department of Pharmacognosy, Medical University of Lublin, 20-093 Lublin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-81448-6350 Simple Summary: Breast cancer (BC) is the most common cancer diagnosed among women world- wide. Despite numerous studies, the pathogenesis of BC is still poorly understood, and effective therapy of this disease remains a challenge for medicine. This article provides the current state of knowledge of the impact of valproic acid (VPA) on different histological subtypes of BC, used in monotherapy or in combination with other active agents in experimental studies in vitro and in vivo. The comprehensive review highlights the progress that has been made on this topic recently. Abstract: Valproic acid (2-propylpentanoic acid, VPA) is a short-chain fatty acid, a member of the group of histone deacetylase inhibitors (HDIs). VPA has been successfully used in the treatment of epilepsy, bipolar disorders, and schizophrenia for over 50 years. Numerous in vitro and in vivo pre-clinical studies suggest that this well-known anticonvulsant drug significantly inhibits cancer cell proliferation by modulating multiple signaling pathways. Breast cancer (BC) is the most common malignancy affecting women worldwide. Despite significant progress in the treatment of BC, serious adverse effects, high toxicity to normal cells, and the occurrence of multi-drug resistance (MDR) Citation: Wawruszak, A.; Halasa, M.; still limit the effective therapy of BC patients. -

Design and Synthesis of Novel Classes of Hdacs and Kmts Inhibitors
University of East Anglia School of Pharmacy Design and synthesis of novel classes of HDACs and KMTs inhibitors by Remy Thomas Narozny Supervisor: Prof. A. Ganesan Second Supervisor: Prof. Mark Searcey Thesis for the degree of Doctor of Philosophy November 2018 This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with the author and that use of any information derived therefrom must be in accordance with current UK Copyright Law. In addition, any quotation or extract must include full attribution. “Your genetics is not your destiny.” George McDonald Church Abstract For long, scientists thought that our body was driven only by our genetic code that we inherited at birth. However, this determinism was shattered entirely and proven as false in the second half of the 21st century with the discovery of epigenetics. Instead, cells turn genes on and off using reversible chemical marks. With the tremendous progression of epigenetic science, it is now believed that we have a certain power over the expression of our genetic traits. Over the years, these epigenetic modifications were found to be at the core of how diseases alter healthy cells, and environmental factors and lifestyle were identified as top influencers. Epigenetic dysregulation has been observed in every major domain of medicine, with a reported implication in cancer development, neurodegenerative pathologies, diabetes, infectious disease and even obesity. Substantially, an epigenetic component is expected to be involved in every human disease. Hence, the modulation of these epigenetics mechanisms has emerged as a therapeutic strategy. -

Histone Deacetylase Inhibitors: a Prospect in Drug Discovery Histon Deasetilaz İnhibitörleri: İlaç Keşfinde Bir Aday
Turk J Pharm Sci 2019;16(1):101-114 DOI: 10.4274/tjps.75047 REVIEW Histone Deacetylase Inhibitors: A Prospect in Drug Discovery Histon Deasetilaz İnhibitörleri: İlaç Keşfinde Bir Aday Rakesh YADAV*, Pooja MISHRA, Divya YADAV Banasthali University, Faculty of Pharmacy, Department of Pharmacy, Banasthali, India ABSTRACT Cancer is a provocative issue across the globe and treatment of uncontrolled cell growth follows a deep investigation in the field of drug discovery. Therefore, there is a crucial requirement for discovering an ingenious medicinally active agent that can amend idle drug targets. Increasing pragmatic evidence implies that histone deacetylases (HDACs) are trapped during cancer progression, which increases deacetylation and triggers changes in malignancy. They provide a ground-breaking scaffold and an attainable key for investigating chemical entity pertinent to HDAC biology as a therapeutic target in the drug discovery context. Due to gene expression, an impending requirement to prudently transfer cytotoxicity to cancerous cells, HDAC inhibitors may be developed as anticancer agents. The present review focuses on the basics of HDAC enzymes, their inhibitors, and therapeutic outcomes. Key words: Histone deacetylase inhibitors, apoptosis, multitherapeutic approach, cancer ÖZ Kanser tedavisi tüm toplum için büyük bir kışkırtıcıdır ve ilaç keşfi alanında bir araştırma hattını izlemektedir. Bu nedenle, işlemeyen ilaç hedeflerini iyileştirme yeterliliğine sahip, tıbbi aktif bir ajan keşfetmek için hayati bir gereklilik vardır. Artan pragmatik kanıtlar, histon deasetilazların (HDAC) kanserin ilerleme aşamasında deasetilasyonu arttırarak ve malignite değişikliklerini tetikleyerek kapana kısıldığını ifade etmektedir. HDAC inhibitörleri, ilaç keşfi bağlamında terapötik bir hedef olarak HDAC biyolojisiyle ilgili kimyasal varlığı araştırmak için, çığır açıcı iskele ve ulaşılabilir bir anahtar sağlarlar. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Histone Deacetylases As New Therapeutic Targets in Triple-Negative Breast Cancer
CANCER GENOMICS & PROTEOMICS 14 : 299-313 (2017) doi:10.21873/cgp.20041 Review Histone Deacetylases as New Therapeutic Targets in Triple- negative Breast Cancer: Progress and Promises NIKOLAOS GARMPIS 1* , CHRISTOS DAMASKOS 1,2* , ANNA GARMPI 3* , EMMANOUIL KALAMPOKAS 4, THEODOROS KALAMPOKAS 5, ELEFTHERIOS SPARTALIS 2, AFRODITE DASKALOPOULOU 2, SERENA VALSAMI 6, MICHAEL KONTOS 7, AFRODITI NONNI 8, KONSTANTINOS KONTZOGLOU 1, DESPINA PERREA 2, NIKOLAOS NIKITEAS 2 and DIMITRIOS DIMITROULIS 1 1Second Department of Propedeutic Surgery, Laiko General Hospital, National and Kapodistrian University of Athens, Medical School, Athens, Greece; 2N.S. Christeas Laboratory of Experimental Surgery and Surgical Research, Medical School, National and Kapodistrian University of Athens, Athens, Greece; 3Internal Medicine Department, Laiko General Hospital, University of Athens Medical School, Athens, Greece; 4Gynaecological Oncology Department, University of Aberdeen, Aberdeen, U.K.; 5Assisted Conception Unit, Second Department of Obstetrics and Gynecology, Aretaieion Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece; 6Blood Transfusion Department, Aretaieion Hospital, Medical School, National and Kapodistrian Athens University, Athens, Greece; 7First Department of Surgery, Laiko General Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece; 8First Department of Pathology, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece Abstract. Triple-negative breast cancer (TNBC) lacks therapies. Given the fact that epigenetic processes control both expression of estrogen receptor (ER), progesterone receptor the initiation and progression of TNBC, there is an increasing (PR) and HER2 gene. It comprises approximately 15-20% of interest in the mechanisms, molecules and signaling pathways breast cancers (BCs). Unfortunately, TNBC’s treatment that participate at the epigenetic modulation of genes continues to be a clinical problem because of its relatively expressed in carcinogenesis. -

Combination of 5-Fluorouracil with Epigenetic Modifiers Induces Radiosensitization, Somatostatin Receptor 2 Expression and Radio
Journal of Nuclear Medicine, published on February 22, 2019 as doi:10.2967/jnumed.118.224048 Combination of 5-Fluorouracil With Epigenetic Modifiers Induces Radiosensitization, Somatostatin Receptor 2 Expression and Radioligand Binding in Neuroendocrine Tumor Cells in Vitro Xi-Feng Jin1#, Christoph J. Auernhammer1,2*, Harun Ilhan 2,3, Simon Lindner 3, Svenja Nölting1,2, Julian Maurer1, Gerald Spöttl1, Michael Orth4,5,6 1 Department of Internal Medicine 4, University-Hospital Campus Grosshadern, Ludwig-Maximilians University of Munich, Munich, Germany 2 Interdisciplinary Center of Neuroendocrine Tumours of the GastroEnteroPancreatic System (GEPNET-KUM), Klinikum der Universitaet Muenchen, Ludwig-Maximilians-University of Munich, Campus Grosshadern, Marchioninistr, 15, 81377, Munich, Germany. 3 Department of Nuclear Medicine, University-Hospital Campus Grosshadern, Ludwig-Maximilians University of Munich, Munich, Germany 4 Department of Radiation Oncology, University Hospital, LMU Munich, Ludwig-Maximilians University of Munich, Munich, Germany 5German Cancer Consortium (DKTK), Munich, Germany 6German Cancer Research Center (DKFZ), Heidelberg, Germany First author# and Corresponding author*: Xi Feng Jin# and Christoph J. Auernhammer*, Department of Internal Medicine 4, University-Hospital Campus Grosshadern, Ludwig-Maximilians University of Munich, Marchioninistr, 15, 81377, Munich, Germany. E-mails: [email protected] / [email protected] 1 All authors have contributed significantly, and all authors are in agreement with the content of the manuscript. Disclosure statements: CJA has received research contracts from Ipsen, Novartis, and ITM Solucin, lecture honorarium from Ipsen, Novartis, and Falk Foundation, and advisory board honorarium from Novartis. SN has received research contracts from Novartis and lecture fees from Ipsen. No other potential conflicts of interest relevant to this article exist. -
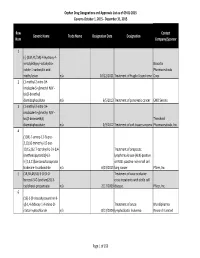
Orphan Drug Designation List
Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 1 (‐)‐(3aR,4S,7aR)‐4‐Hydroxy‐4‐ m‐tolylethynyl‐octahydro‐ Novartis indole‐1‐carboxylic acid Pharmaceuticals methyl ester n/a 10/12/2011 Treatment of Fragile X syndrome Corp. 2 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐broethyl) diamidophosphate n/a 6/5/2013 Treatment of pancreatic cancer EMD Serono 3 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐bromoethyl) Threshold diamidophosphate n/a 3/9/2012 Treatment of soft tissue sarcoma Pharmaceuticals, Inc. 4 (1OR)‐7‐amino‐12‐fluoro‐ 2,10,16‐trimethyl‐15 oxo‐ 10,15,16,17‐tetrahydro‐2H‐8,4‐ Treatment of anaplastic (metheno)pyrazolo[4,3‐ lymphoma kinase (ALK)‐positive h][2,5,11]benzoxadiazacyclote or ROS1‐positive non‐small cell tradecine‐3‐carbonitrile n/a 6/23/2015 lung cancer Pfizer, Inc. 5 (1R,3R,4R,5S)‐3‐O‐[2‐O‐ Treatment of vaso‐occlusive benzoyl‐3‐O‐(sodium(2S)‐3‐ crisis in patients with sickle cell cyclohexyl‐propanoate‐ n/a 2/17/2009 disease. Pfizer, Inc. 6 (1S)‐1‐(9‐deazahypoxanthin‐9‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ Treatment of acute Mundipharma ribitol‐hydrochloride n/a 8/13/2004 lymphoblastic leukemia Research Limited Page 1 of 359 Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 7 Treatment of chronic lymphocytic leukemia and related leukemias to include (1S)‐1‐(9‐deazahypoxanthin‐9‐ prolymphocytic leukemia, adult T‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ cell leukemia, and hairy cell Mundipharma ribitol‐hydrochloride n/a 8/10/2004 leukemia Research Ltd. -

Histone Deacetylase Inhibitors As Anticancer Drugs
International Journal of Molecular Sciences Review Histone Deacetylase Inhibitors as Anticancer Drugs Tomas Eckschlager 1,*, Johana Plch 1, Marie Stiborova 2 and Jan Hrabeta 1 1 Department of Pediatric Hematology and Oncology, 2nd Faculty of Medicine, Charles University and University Hospital Motol, V Uvalu 84/1, Prague 5 CZ-150 06, Czech Republic; [email protected] (J.P.); [email protected] (J.H.) 2 Department of Biochemistry, Faculty of Science, Charles University, Albertov 2030/8, Prague 2 CZ-128 43, Czech Republic; [email protected] * Correspondence: [email protected]; Tel.: +42-060-636-4730 Received: 14 May 2017; Accepted: 27 June 2017; Published: 1 July 2017 Abstract: Carcinogenesis cannot be explained only by genetic alterations, but also involves epigenetic processes. Modification of histones by acetylation plays a key role in epigenetic regulation of gene expression and is controlled by the balance between histone deacetylases (HDAC) and histone acetyltransferases (HAT). HDAC inhibitors induce cancer cell cycle arrest, differentiation and cell death, reduce angiogenesis and modulate immune response. Mechanisms of anticancer effects of HDAC inhibitors are not uniform; they may be different and depend on the cancer type, HDAC inhibitors, doses, etc. HDAC inhibitors seem to be promising anti-cancer drugs particularly in the combination with other anti-cancer drugs and/or radiotherapy. HDAC inhibitors vorinostat, romidepsin and belinostat have been approved for some T-cell lymphoma and panobinostat for multiple myeloma. Other HDAC inhibitors are in clinical trials for the treatment of hematological and solid malignancies. The results of such studies are promising but further larger studies are needed.