Insights Into Equilibrium and Adsorption Rate of Phenol on Activated Carbon Pellets Derived from Cigarette Butts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Japanese Sunflower Tithonia Diversifolia
Invasive plant Japanese sunflower Tithonia diversifolia Japanese sunflower is native to Central America. It is a Legal requirements serious environmental weed, forming dense thickets and out-competing native vegetation. Japanese sunflower is not a prohibited or restricted invasive plant under the Biosecurity Act 2014. However, Japanese sunflower is commonly a weed on roadsides by law, everyone has a general biosecurity obligation and embankments in coastal Queensland and northern (GBO) to take reasonable and practical steps to minimise coastal New South Wales. It is widespread and common the risks associated with invasive plants under their in far north Queensland, particularly on roadsides, control. embankments, unmanaged lands and fire degraded hillsides. Local governments must have a biosecurity plan that A similar species Tithonia rotundifolia is known as Mexican covers invasive plants in their area. This plan may include sunflower. This weed is smaller in height and flower size, actions to be taken on certain species. Some of these and its distribution as an environmental weed is similar to actions may be required under local laws. Contact your Japanese sunflower in Queensland but not as common in local government for more information. New South Wales. Description situations specified on the product labels. A permit held by the Department of Agriculture and Fisheries allows Japanese sunflower stands up to to 3 m. Flowers are people generally to use some herbicide products to control sunflower-like heads up to 10 cm across, with yellow flower Japanese sunflower as an environmental weed in various centres and reddish-orange petals 4–5 cm long. The stems situations. -

Sfblake and Tithonia Diversifolia
Prospective agents for the biological control of Tithonia rotundifolia (Mill.) S.F.Blake and Tithonia diversifolia (Hemsl.) A.Gray (Asteraceae) in South Africa D.O. Simelane1*, K.V. Mawela1 & A. Fourie2 1Agricultural Research Council-Plant Protection Research Institute, Private Bag X134, Queenswood, 0121 South Africa 2Agricultural Research Council-Plant Protection Research Institute, Private Bag X5017 Stellenbosch, 7599 South Africa Starting in 2007, two weedy sunflower species, Tithonia rotundifolia (Mill.) S.F.Blake and Tithonia diversifolia (Hemsl.) A.Gray (Asteraceae: Heliantheae), were targeted for biological control in South Africa. Surveys conducted in their native range (Mexico) revealed that there were five potential biological control agents for T.rotundifolia, and three of these are currently undergoing host-specificity and performance evaluations in South Africa. Two leaf-feeding beetles, Zygogramma signatipennis (Stål) and Zygogramma piceicollis (Stål) (Coleoptera: Chrysomelidae), are the most promising biological control agents for T. rotundifolia: prelimi- nary host-specificity trials suggest that they are adequately host-specific. The stem-boring beetle, Lixus fimbriolatus Boheman (Coleoptera: Curculionidae), is also highly damaging to T. rotundifolia, but its host range is yet to be determined. Two other stem-boring beetles, Canidia mexicana Thomson (Coleoptera: Cerambycidae) and Rhodobaenus auctus Chevrolat (Coleoptera: Curculionidae), have also been recorded on T. rotundifolia, and these will be considered for further testing if L. fimbriolatus is found to be unsuitable for release in South Africa. Only two insect species were imported as candidate agents on T. diversifolia, the leaf-feeding butterfly Chlosyne sp. (Lepidoptera: Nymphalidae), and an unidentified stem-boring moth (Lepidoptera: Tortricidae): the latter was tested in quarantine but rejected because it attacked several sunflower cultivars. -

Enhancing Archaeoparasitology By
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE DIGGING DEEPER: ENHANCING ARCHAEOPARASITOLOGY BY COMBINING MOLECULAR METHODS WITH TRADITIONAL MORPHOLOGICAL APPROACHES A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY By LAUREN MARIE CLEELAND Norman, Oklahoma 2015 DIGGING DEEPER: ENHANCING ARCHAEOPARASITOLOGY BY COMBINING MOLECULAR METHODS WITH TRADITIONAL MORPHOLOGICAL APPROACHES A DISSERTATION APPROVED FOR THE DEPARTMENT OF ANTHROPOLOGY BY ______________________________ Dr. Cecil M. Lewis, Co-Chair ______________________________ Dr. Susan C. Vehik, Co-Chair ______________________________ Dr. Tassie Hirschfeld ______________________________ Dr. Patrick Livingood ______________________________ Dr. Paul Lawson © Copyright by LAUREN MARIE CLEELAND 2015 All Rights Reserved. I dedicate this dissertation to the memory of my husband, Peter Riley Cleeland (1966-2012). Acknowledgements I offer my deepest gratitude to the members of my committee, who have encouraged and supported me through the doctoral process. Especially, I thank Dr. Cecil Lewis and Dr. Susan Vehik for their guidance and the gift of their knowledge, and supporting me through the loss of my husband, which delayed this process. I thank Dr. Tassie Hirschfeld for her insight and direction in thinking about the modern implications of prehistoric parasite work. I thank Dr. Patrick Livingood for his wisdom and enthusiasm and his willingness to always discuss a variety of topics. I thank Dr. Paul Lawson for bringing a broader ecological consideration to the study of prehistoric parasitism. I thank the University of Oklahoma Graduate School for providing the opportunity for me to study and gain the experience I need to master the concepts that have culminated in this dissertation. I thank the Department of Anthropology for their support and guidance. -

Mexican Sunflower, Tithonia Rotundifolia
A Horticulture Information article from the Wisconsin Master Gardener website, posted 6 Aug 2018 Mexican sunfl ower, Tithonia rotundifolia The genus Tithonia in the daisy family (Asteraceae) includes 10-15 species of bushy annuals, perennials and shrubs native to Mexico and Central America that have large, brightly colored daisy-like fl owers on thick stems. Mexican sunfl ower, T. rotundifolia, is a vigorous, drought tolerant warm season annual that is easy to grow in the ornamental garden with other common names of red sunfl ower of just tithonia. Tithonia plants grow 4-6+ feet tall, with a large central stalk and a somewhat gangly branching habit. The stems can be brittle. The dark green leaves are ovate to deltoid (triangular) in shape, with serrate to crenate margins. The coarse leaves are usually entire but occasionally will be three lobed. Mexican sunfl ower, Tithonia rotundifolia, is a tall plant. The foliage and stems are covered with a soft downy fuzz, and the underside of The foliage of Mexican sunfl ower is coarse and hairy (L); the ovoid to deltoid the leaves are hairy. leaves have serrate margins and are usually entire (C) but may be three lobed (R). Flowers are produced from mid-summer until frost. The solitary fl owers are borne on fragile hollow peduncles (fl ower stems) that are susceptible to being bent and are often broken by birds. Each 3-inch blossom has a number of bright red to orange ray fl owers surrounding the central yellow disk fl owers. Thick, fuzzy buds (L) open (LC) to reveal bright red to orange ray fl owers (C) surrounding yellow disk fl owers (RC) that remain for a while after the ray fl owers fall off (R). -

Revision of the Genus Tithonia •. • •
.;. .• • • REVISION OF THE GENUS TITHONIA •. • • By S. F. BLAKE. INTRODUCTION. The genus Tith01lia, originally described in 1789 in Jussieu's Genera 1 without citation of species, was adopted by J. F. Gmelin' two years later, and the single known species was given the binomial T. unijWra, a name which has been universally displaced by the later Tithooia tagetijlqra, published by Desfontaines in 1802 with a full description and plate. The same plant, grown by Philip Mi lIer in his Chelsea garden from seed sent presumably from Veracruz by William Houstoun, had been described in the eighth edition of the Gardeners' Dictionary in 1768 as Tagetes rotundifolia, and as this is the earliest binomial given the species it must now be known as Tithonia rOflundifolia. It is a showy annual with large, orange or golden-yellow heads, much like the common sunflower in appearance except for the yellow disk, and seems worthy the attention of horticulturists. As here recognized, the genus Tithooia includes ten species, native from northern Mexico to Panama. One species, T. rotundifolia, occurs also in the Greater and Lesser Antilles, and in Venezuela (where certainly introduced), and another, T. diversifolia, has become a weed in Ceylon and Burma and at Singapore. As the relationships of the genus to Helianthus and Viguiera have already been COn sidered in some detail by the writer in another publication,' only brief notice of them is necessary here. The typical pappus-bearing members of the genus are separated from H elianthU8 by their per sistent pappus of awns and squamellae, and from Viguiera chiefly by their fistulose peduncles and by certain details of involucre. -

Flórula Vascular De La Sierra De Catorce Y Territorios Adyacentes, San Luis Potosi, México
Acta Botanica Mexicana 78: 1-38 (2007) FLÓRULA VASCULAR DE LA SIERRA DE CATORCE Y TERRITORIOS ADYACENTES, SAN LUIS POTOSI, MÉXICO ONÉSIMO GONZÁLEZ COSTILLA1,2, JOAQUÍN GIMÉNEZ DE AZCÁRATE3, JOSÉ GARCÍA PÉREZ1 Y JUAN RogELIO AGUIRRE RIVERA1 1Universidad Autónoma de San Luis Potosí, Instituto de Investigación de Zonas Desérticas, Altair 200, Fraccionamiento El Llano, Apdo. postal 504, 78377 San Luis Potosí, México. 2Universidad Complutense de Madrid, Departamento de Biología Vegetal II, Facultad de Farmacia, Madrid, España. [email protected] 3Universidad de Santiago de Compostela, Departamento de Botánica, Escuela Politécnica Superior, 27002 Lugo, España. RESUMEN La Sierra de Catorce, localizada en el norte del estado de San Luis Potosí, reúne algunas de las principales cimas del Desierto Chihuahuense cuyas cotas superan los 3000 metros. Ello ha favorecido que la Sierra sea una importante área de diversificación de la flora y las fitocenosis de dicha ecorregión. A partir del estudio fitosociológico de la vegetación del territorio, que se está realizando desde 1999, se ha obtenido un catálogo preliminar de su flora. Hasta el momento la lista de plantas vasculares está conformada por 526 especies y cuatro taxa infraespecíficos, agrupados en 293 géneros y 88 familias. Las familias y géneros mejor representados son Asteraceae, Poaceae, Cactaceae, Fabaceae, Fagaceae y Lamiaceae, así como Quercus, Opuntia, Muhlenbergia, Salvia, Agave, Bouteloua y Dyssodia, respectivamente. Asimismo se señalan los tipos de vegetación representativos del área que albergan los diferentes taxa. Por último, con base en diferentes listas de flora amenazada, se identificaron las especies incluidas en alguna de las categorías reconocidas. Palabras clave: Desierto Chihuahuense, estudio fitosociológico, flora, flora ame- nazada, México, San Luis Potosí, Sierra de Catorce. -

The Native Mexican Sunflower
The Native Mexican Sunflower By Ray Novitske, Fairfax Master Gardener Tithonia, also known as a Mexican sunflower, sounds more like a city in Michigan or an eastern European country than a native Mexican sunflower. The plant was named for Tithonus, a man loved by Aurora, goddess of the dawn in Roman mythology. Of the two major species, Tithonia diversifolia and Tithonia rotundifolia, I am growing the later for the first time this summer. There are a handful of popular cultivars available such as ‘Fiesta del Sol,’ ‘Goldfinger,’ and ‘Torch.’ This member of the Aster family is a native of Mexico and Central America. It appealed to me because of its by author by sizzling orange flowers and its large stately presence in a garden that can’t be missed. It is noted as a pollinator photo: photo: magnet, attracting hummingbirds because of its color, along with bees and butterflies for the pollen. While Tithonia rotundifolia tending to my Tithonia, I swatted away a hummingbird from my head, initially thinking it was a bee. The bumblebees prefer my Echinops and Echinacea, but the small bees adore the Tithonia. I planted these from seed indoors and transplanted them into my heat garden along the sunny south-facing side of a brick house. They are drought tolerant, prefer full sun and average soil and love hot weather. Rich soil tends to produce weak stems and lush foliage at the expense of the flowers. They are fast growers, and now stand at 5 feet (1.5 m) tall in my garden. I have them growing in average but well-drained soil. -

Forsskål and the Interpretation of Article 23 Author(S): C
Forsskål and the Interpretation of Article 23 Author(s): C. Jeffrey Reviewed work(s): Source: Taxon, Vol. 34, No. 1 (Feb., 1985), pp. 144-147 Published by: International Association for Plant Taxonomy (IAPT) Stable URL: http://www.jstor.org/stable/1221579 . Accessed: 23/07/2012 03:34 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. International Association for Plant Taxonomy (IAPT) is collaborating with JSTOR to digitize, preserve and extend access to Taxon. http://www.jstor.org FORSSKAL AND THE INTERPRETATION OF ARTICLE 23 C. Jeffrey' Summary Article 23 is shown to be equivocal with respectto the validity of specificnames publishedin the 'FloraAegyptiaco-Arabica' of ForsskAl(1775) and similarworks. Burdetand Perret(1983) have concludedthat, under the provisionsofArt. 23.6(c)of the International Code of BotanicalNomenclature (Voss et al., 1983), all the specificnames first publishedin certain worksof Asso, Aublet,Forsskil and Grimmmust be regardedas not validly published,on the grounds thatthese areworks in whichthe Linnaeansystem of binarynomenclature for speciesis not consistently employed.Their interpretation of this Articleis supportedby Friiset al. (1984), who rightlyemphasize the undesirablenomenclatural consequences of such a conclusion and invite comments as to how they might best be avoided. -

Sundiversifolide from Exudates of Mexican Sunflower (Tithonia Diversifolia (Hemsl.) A
Eco-Engineering, 18(2), 77-81, 2006 Original Paper Sundiversifolide from Exudates of Mexican Sunflower (Tithonia diversifolia (Hemsl.) A. Gray) Achenes Takako Kato*, Masamichi Yamashita**, Koji Hasegawa* and Kaori Tomita-Yokotani* * Doctoral Program in Life Sciences and Bioengineering, University of Tsukuba Tsukuba, Ibaraki 305-8572, Japan ** Institute of Space and Astronautical Science /JAXA Yoshinodai, Sagamihara, Kanagawa 229-8510, Japan (Received December 20, 2005; Accepted March 22, 2006) ABSTRACT The purpose of this study was to confirm the existence of sundiversifolide, 4,15-dinor-3-hydroxy-1(5)-xanthene- 12,8-olide, as an allelopathic substance in two species of plants. This substance could be used to control an ecosystem by its allelopathic function. Sundiversifolide has species-specific allelopathic properties in germinating seeds (achenes) of the sunflower, Helianthus annuus L.cv. Taiyo. However, its distribution among other species of plants has not yet been elucidated. The allelopathic properties and the identification of their substances were investigated in the exudates from the achenes of the Mexican sunflower (Tithonia diversifolia (Hemsl.) A. Gray) and Leucanthemum paludosum cv. North Pole. In the exudates from the Mexican sunflower achens, the existence of sundiversifolide was identified by LC- ESI+/MS. In the exudates from the L. paludosum, sundiversifolide was not detected. The low polar fraction of the exudates from the achenes of the Mexican sunflower has an allelopathic function as does the sunflower plant. Allelopathic properties of achenes of Mexican sunflower was also examined. The ethylacetate-soluble fraction of the exudates inhibited the growth of cat’s-eyes (Veronica persica Poiret) seedlings and the conidial germination of useful fungi, i.e. -

Threats to Australia's Grazing Industries by Garden
final report Project Code: NBP.357 Prepared by: Jenny Barker, Rod Randall,Tony Grice Co-operative Research Centre for Australian Weed Management Date published: May 2006 ISBN: 1 74036 781 2 PUBLISHED BY Meat and Livestock Australia Limited Locked Bag 991 NORTH SYDNEY NSW 2059 Weeds of the future? Threats to Australia’s grazing industries by garden plants Meat & Livestock Australia acknowledges the matching funds provided by the Australian Government to support the research and development detailed in this publication. This publication is published by Meat & Livestock Australia Limited ABN 39 081 678 364 (MLA). Care is taken to ensure the accuracy of the information contained in this publication. However MLA cannot accept responsibility for the accuracy or completeness of the information or opinions contained in the publication. You should make your own enquiries before making decisions concerning your interests. Reproduction in whole or in part of this publication is prohibited without prior written consent of MLA. Weeds of the future? Threats to Australia’s grazing industries by garden plants Abstract This report identifies 281 introduced garden plants and 800 lower priority species that present a significant risk to Australia’s grazing industries should they naturalise. Of the 281 species: • Nearly all have been recorded overseas as agricultural or environmental weeds (or both); • More than one tenth (11%) have been recorded as noxious weeds overseas; • At least one third (33%) are toxic and may harm or even kill livestock; • Almost all have been commercially available in Australia in the last 20 years; • Over two thirds (70%) were still available from Australian nurseries in 2004; • Over two thirds (72%) are not currently recognised as weeds under either State or Commonwealth legislation. -

Productive and Nutritional Aspects of Tithonia Diversifolia Fertilized with Biofertilizer and Irrigated
Journal of Agricultural Science; Vol. 10, No. 11; 2018 ISSN 1916-9752 E-ISSN 1916-9760 Published by Canadian Center of Science and Education Productive and Nutritional Aspects of Tithonia diversifolia Fertilized With Biofertilizer and Irrigated Matheus M. Reis1,2, Leonardo D. Tuffi Santos2, Rodinei F. Pegoraro2, Marcia V. Santos3, Fernando Colen2, William G. Montes2, Ronie R. Moura Jr.2, Leandro R. da Cruz4 & Flávio G. Oliveira2 1 School of Agricultural Engineering, University of Campinas, Campinas, Brazil 2 Institute of Agrarian Sciences, Federal University of Minas Gerais, Montes Claros, Brazil 3 School of Agrarian Sciences, Federal University of Vales do Jequitinhonha e Mucuri, Diamantina, Brazil 4 School of Agriculture, São Paulo State University, Botucatu, Brazil Correspondence: Matheus M. Reis, Institute of Agrarian Sciences, Federal University of Minas Gerais, Montes Claros, Minas Gerais, Brazil. Tel: 55-038-2101-7781. E-mail: [email protected] Received: July 24, 2018 Accepted: August 26, 2018 Online Published: October 15, 2018 doi:10.5539/jas.v10n11p367 URL: https://doi.org/10.5539/jas.v10n11p367 Abstract Little is known about the agronomic aspects of Mexican Sunflower (Tithonia diversifolia), in spite of its potential for multiple uses. In this study, we evaluated the effects of application rates of biofertilizer and irrigation on yield, growth, and leaf chlorophyll and nutrient content of Mexican Sunflower. In an experiment in the Brazilian semi-arid region, we used a 5 × 2 factorial arrangement, consisting of five application rates of biofertilizer (0, 40, 80, 120, and 160 m3 ha-1), with and without irrigation. The statistical design was randomized blocks with three replications. -
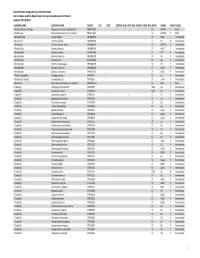
Element Status Designations by Common Name Arizona Game And
Element Status Designations by Common Name Arizona Game and Fish Department, Heritage Data Management System Updated: 10/15/2019 COMMON NAME SCIENTIFIC NAME ELCODE ESA DATE CRITHAB BLM USFS NESL MEXFED SGCN NPL SRANK GRANK TRACK TAXON A Arizona‐Mexican Orange Choisya arizonica var. amplophylla PDRUT02031 S2 G4TNR Y Plant A Balsamroot Balsamorhiza hookeri var. hispidula PDAST11041 S1 G5T3T5 Y Plant A Blueberry Bee Osmia ribifloris IIHYMA2570 S? G4G5 Y Invertebrate A Buckmoth Hemileuca grotei IILEW0M070 S? G4 N Invertebrate A Buckmoth Hemileuca grotei diana IILEW0M072 S? G4T3T4 Y Invertebrate A Bumble Bee Bombus centralis IIHYM24100 S? G4G5 Y Invertebrate A Bumble Bee Bombus fervidus IIHYM24110 S? G4? Y Invertebrate A Bumble Bee Bombus flavifrons IIHYM24120 S? G5 Y Invertebrate A Bumble Bee Bombus huntii IIHYM24140 S? G5 Y Invertebrate A Bumble Bee Bombus melanopygus IIHYM24150 S? G5 Y Invertebrate A Bumble Bee Bombus morrisoni IIHYM24460 S? G4G5 Y Invertebrate A Bumble Bee Bombus nevadensis IIHYM24170 S? G4G5 Y Invertebrate A Bushtail Caddisfly Gumaga griseola IITRI53010 S? G5 Y Invertebrate A Bushtailed Caddisfly Gumaga nigricula IITRI53020 S? G3G4 Y Invertebrate A Buttercup Ranunculus inamoenus var. subaffinis PDRAN0L1C3 S1 G5T1 YPlant A caddisfly Hydropsyche occidentalis IITRI25460 S2S3 G5 Y Invertebrate A caddisfly Hydropsyche oslari IITRIG6010 S2S3 G5 Y Invertebrate A caddisfly Lepidostoma apache IITRI64A10 S S1 G1 Y Invertebrate A Caddisfly Agapetus boulderensis IITRI33190 S? G5 Y Invertebrate A Caddisfly Alisotrichia arizonica IITRID7010