Gulf of Alaska Groundfish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOTES on the BIRDS of CHIRIKOF ISLAND, ALASKA Jack J
NOTES ON THE BIRDS OF CHIRIKOF ISLAND, ALASKA JACK J. WITHROW, University of Alaska Museum, 907 Yukon Drive, Fairbanks, Alaska 99775; [email protected] ABSTRACT: Isolated in the western Gulf of Alaska 61 km from nearest land and 74 km southwest of the Kodiak archipelago, Chirikof Island has never seen a focused investigation of its avifauna. Annotated status and abundance for 89 species recorded during eight visits 2008–2014 presented here include eastern range extensions for three Beringian subspecies of the Pacific Wren (Troglodytes pacificus semidiensis), Song Sparrow (Melospiza melodia sanaka), and Gray-crowned Rosy-Finch (Leucost- icte tephrocotis griseonucha). A paucity of breeding bird species is thought to be a result of the long history of the presence of introduced cattle and introduced foxes (Vulpes lagopus), both of which persist to this day. Unique among sizable islands in southwestern Alaska, Chirikof Island (55° 50′ N 155° 37′ W) has escaped focused investigations of its avifauna, owing to its geographic isolation, lack of an all-weather anchorage, and absence of major seabird colonies. In contrast, nearly every other sizable island or group of islands in this region has been visited by biologists, and they or their data have added to the published literature on birds: the Aleutian Is- lands (Gibson and Byrd 2007), the Kodiak archipelago (Friedmann 1935), the Shumagin Islands (Bailey 1978), the Semidi Islands (Hatch and Hatch 1983a), the Sandman Reefs (Bailey and Faust 1980), and other, smaller islands off the Alaska Peninsula (Murie 1959, Bailey and Faust 1981, 1984). With the exception of most of the Kodiak archipelago these islands form part of the Alaska Maritime National Wildlife Refuge (AMNWR), and many of these publications are focused largely on seabirds. -
Schedule Onlinepdf
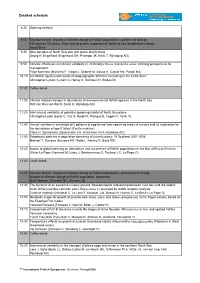
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

California Saltwater Sport Fishing Regulations
2017–2018 CALIFORNIA SALTWATER SPORT FISHING REGULATIONS For Ocean Sport Fishing in California Effective March 1, 2017 through February 28, 2018 13 2017–2018 CALIFORNIA SALTWATER SPORT FISHING REGULATIONS Groundfish Regulation Tables Contents What’s New for 2017? ............................................................. 4 24 License Information ................................................................ 5 Sport Fishing License Fees ..................................................... 8 Keeping Up With In-Season Groundfish Regulation Changes .... 11 Map of Groundfish Management Areas ...................................12 Summaries of Recreational Groundfish Regulations ..................13 General Provisions and Definitions ......................................... 20 General Ocean Fishing Regulations ��������������������������������������� 24 Fin Fish — General ................................................................ 24 General Ocean Fishing Fin Fish — Minimum Size Limits, Bag and Possession Limits, and Seasons ......................................................... 24 Fin Fish—Gear Restrictions ................................................... 33 Invertebrates ........................................................................ 34 34 Mollusks ............................................................................34 Crustaceans .......................................................................36 Non-commercial Use of Marine Plants .................................... 38 Marine Protected Areas and Other -

The Exchange of Water Between Prince William Sound and the Gulf of Alaska Recommended
The exchange of water between Prince William Sound and the Gulf of Alaska Item Type Thesis Authors Schmidt, George Michael Download date 27/09/2021 18:58:15 Link to Item http://hdl.handle.net/11122/5284 THE EXCHANGE OF WATER BETWEEN PRINCE WILLIAM SOUND AND THE GULF OF ALASKA RECOMMENDED: THE EXCHANGE OF WATER BETWEEN PRIMCE WILLIAM SOUND AND THE GULF OF ALASKA A THESIS Presented to the Faculty of the University of Alaska in partial fulfillment of the Requirements for the Degree of MASTER OF SCIENCE by George Michael Schmidt III, B.E.S. Fairbanks, Alaska May 197 7 ABSTRACT Prince William Sound is a complex fjord-type estuarine system bordering the northern Gulf of Alaska. This study is an analysis of exchange between Prince William Sound and the Gulf of Alaska. Warm, high salinity deep water appears outside the Sound during summer and early autumn. Exchange between this ocean water and fjord water is a combination of deep and intermediate advective intrusions plus deep diffusive mixing. Intermediate exchange appears to be an annual phen omenon occurring throughout the summer. During this season, medium scale parcels of ocean water centered on temperature and NO maxima appear in the intermediate depth fjord water. Deep advective exchange also occurs as a regular annual event through the late summer and early autumn. Deep diffusive exchange probably occurs throughout the year, being more evident during the winter in the absence of advective intrusions. ACKNOWLEDGMENTS Appreciation is extended to Dr. T. C. Royer, Dr. J. M. Colonell, Dr. R. T. Cooney, Dr. R. -

Bathymetry and Geomorphology of Shelikof Strait and the Western Gulf of Alaska
geosciences Article Bathymetry and Geomorphology of Shelikof Strait and the Western Gulf of Alaska Mark Zimmermann 1,* , Megan M. Prescott 2 and Peter J. Haeussler 3 1 National Marine Fisheries Service, Alaska Fisheries Science Center, National Oceanic and Atmospheric Administration (NOAA), Seattle, WA 98115, USA 2 Lynker Technologies, Under contract to Alaska Fisheries Science Center, Seattle, WA 98115, USA; [email protected] 3 U.S. Geological Survey, 4210 University Dr., Anchorage, AK 99058, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-206-526-4119 Received: 22 May 2019; Accepted: 19 September 2019; Published: 21 September 2019 Abstract: We defined the bathymetry of Shelikof Strait and the western Gulf of Alaska (WGOA) from the edges of the land masses down to about 7000 m deep in the Aleutian Trench. This map was produced by combining soundings from historical National Ocean Service (NOS) smooth sheets (2.7 million soundings); shallow multibeam and LIDAR (light detection and ranging) data sets from the NOS and others (subsampled to 2.6 million soundings); and deep multibeam (subsampled to 3.3 million soundings), single-beam, and underway files from fisheries research cruises (9.1 million soundings). These legacy smooth sheet data, some over a century old, were the best descriptor of much of the shallower and inshore areas, but they are superseded by the newer multibeam and LIDAR, where available. Much of the offshore area is only mapped by non-hydrographic single-beam and underway files. We combined these disparate data sets by proofing them against their source files, where possible, in an attempt to preserve seafloor features for research purposes. -

Fishery Management Plan for Groundfish of the Bering Sea and Aleutian Islands Management Area APPENDICES
FMP for Groundfish of the BSAI Management Area Fishery Management Plan for Groundfish of the Bering Sea and Aleutian Islands Management Area APPENDICES Appendix A History of the Fishery Management Plan ...................................................................... A-1 A.1 Amendments to the FMP ......................................................................................................... A-1 Appendix B Geographical Coordinates of Areas Described in the Fishery Management Plan ..... B-1 B.1 Management Area, Subareas, and Districts ............................................................................. B-1 B.2 Closed Areas ............................................................................................................................ B-2 B.3 PSC Limitation Zones ........................................................................................................... B-18 Appendix C Summary of the American Fisheries Act and Subtitle II ............................................. C-1 C.1 Summary of the American Fisheries Act (AFA) Management Measures ............................... C-1 C.2 Summary of Amendments to AFA in the Coast Guard Authorization Act of 2010 ................ C-2 C.3 American Fisheries Act: Subtitle II Bering Sea Pollock Fishery ............................................ C-4 Appendix D Life History Features and Habitat Requirements of Fishery Management Plan SpeciesD-1 D.1 Walleye pollock (Theragra calcogramma) ............................................................................ -

Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries
JANUARY 2020 Wholesale Market Profiles for Alaska Groundfish and FisheriesCrab Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries JANUARY 2020 JANUARY Prepared by: McDowell Group Authors and Contributions: From NOAA-NMFS’ Alaska Fisheries Science Center: Ben Fissel (PI, project oversight, project design, and editor), Brian Garber-Yonts (editor). From McDowell Group, Inc.: Jim Calvin (project oversight and editor), Dan Lesh (lead author/ analyst), Garrett Evridge (author/analyst) , Joe Jacobson (author/analyst), Paul Strickler (author/analyst). From Pacific States Marine Fisheries Commission: Bob Ryznar (project oversight and sub-contractor management), Jean Lee (data compilation and analysis) This report was produced and funded by the NOAA-NMFS’ Alaska Fisheries Science Center. Funding was awarded through a competitive contract to the Pacific States Marine Fisheries Commission and McDowell Group, Inc. The analysis was conducted during the winter of 2018 and spring of 2019, based primarily on 2017 harvest and market data. A final review by staff from NOAA-NMFS’ Alaska Fisheries Science Center was completed in June 2019 and the document was finalized in March 2016. Data throughout the report was compiled in November 2018. Revisions to source data after this time may not be reflect in this report. Typically, revisions to economic fisheries data are not substantial and data presented here accurately reflects the trends in the analyzed markets. For data sourced from NMFS and AKFIN the reader should refer to the Economic Status Report of the Groundfish Fisheries Off Alaska, 2017 (https://www.fisheries.noaa.gov/resource/data/2017-economic-status-groundfish-fisheries-alaska) and Economic Status Report of the BSAI King and Tanner Crab Fisheries Off Alaska, 2018 (https://www.fisheries.noaa. -

Rockfish (Sebastes) That Are Evolutionarily Isolated Are Also
Biological Conservation 142 (2009) 1787–1796 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Rockfish (Sebastes) that are evolutionarily isolated are also large, morphologically distinctive and vulnerable to overfishing Karen Magnuson-Ford a,b, Travis Ingram c, David W. Redding a,b, Arne Ø. Mooers a,b,* a Biological Sciences, Simon Fraser University, 8888 University Drive, Burnaby BC, Canada V5A 1S6 b IRMACS, Simon Fraser University, 8888 University Drive, Burnaby BC, Canada V5A 1S6 c Department of Zoology and Biodiversity Research Centre, University of British Columbia, #2370-6270 University Blvd., Vancouver, Canada V6T 1Z4 article info abstract Article history: In an age of triage, we must prioritize species for conservation effort. Species more isolated on the tree of Received 23 September 2008 life are candidates for increased attention. The rockfish genus Sebastes is speciose (>100 spp.), morpho- Received in revised form 10 March 2009 logically and ecologically diverse and many species are heavily fished. We used a complete Sebastes phy- Accepted 18 March 2009 logeny to calculate a measure of evolutionary isolation for each species and compared this to their Available online 22 April 2009 morphology and imperilment. We found that evolutionarily isolated species in the northeast Pacific are both larger-bodied and, independent of body size, morphologically more distinctive. We examined Keywords: extinction risk within rockfish using a compound measure of each species’ intrinsic vulnerability to Phylogenetic diversity overfishing and categorizing species as commercially fished or not. Evolutionarily isolated species in Extinction risk Conservation priorities the northeast Pacific are more likely to be fished, and, due to their larger sizes and to life history traits Body size such as long lifespan and slow maturation rate, they are also intrinsically more vulnerable to overfishing. -

Population Assessment, Ecology and Trophic Relationships of Steller Sea Liorq in the Gulf of Alaska
Al'lNUAL REPORT Contract #03-5-022-69 Research Unit #243 1 April 1978 - 31 Marc~ 1979 Pages: Population Assessment, Ecology and Trophic Relationships of Steller Sea LiorQ in the Gulf of Alaska Principal Investigators: Donald Calkins, Marine Mammal Biologist Kenneth Pitcher, Marine ~~mmal Biologist Alaska Department of Fish and Game 333 Raspberry Road Anchorage, Alaska 99502 Assisted by: Karl Schneider Dennis McAllister Walt Cunningham Susan Stanford Dave Johnson Louise Smith Paul Smith Nancy Murray TABLE OF CONTENTS Page Introduction. • . i Steller Sea lions in the Gulf of Alaska (by Donald Calkins). 3 Breeding Rookeries and Hauling Areas . 3 I. Surveys • . 3 II. Pup counts. 4 Distribution and Movements . 10 Sea Otter Distribution and Abundance in the Southern Kodiak Archipelago and the Semidi Islands (by Karl Schneider). • • • • 24 Summary •••••• 24 Introduction • • • 25 Kodiak Archipelago 26 Background • • • • . 26 Methods•.•••. 27 Results and discussion • • • • • . 28 1. Distribution••• 30 2. Population size •••••. 39 3. Status. 40 4. Future. 41 Semidi Islands 43 Background • • 43 Methods ••••••••• 43 Results and discussion • 44 Belukha Whales in Lower Cook Inlet (by Nancy Murray) . • • • • • 47 Distribution and Abundance • 47 Habitat••..•••• 50 Population Dynamics •• 54 Food Habits•••••.••. 56 Behavior • . 58 Literature Cited. 59 Introduction This project is a detailed study of the population dynamics, life history and some aspects of the ecology of the Steller sea lion (Eumetopias jubatus). In addition to the sea lion investigations, the work has been expanded to include an examination of the distribution and abundance of belukha whales (Detphinapterus Zeu~dS) in Cook Inlet and the distribution and abundance of sea otters (Enhydra lutris) near the south end of the Kodiak Archipelago. -

Management Plan for the Yelloweye Rockfish (Sebastes Ruberrimus) in Canada
Proposed Species at Risk Act Management Plan Series Management Plan for the Yelloweye Rockfish (Sebastes ruberrimus) in Canada Yelloweye Rockfish 2018 Recommended citation: Fisheries and Oceans Canada. 2018. Management Plan for the Yelloweye Rockfish (Sebastes ruberrimus) in Canada [Proposed]. Species at Risk Act Management Plan Series. Fisheries and Oceans Canada, Ottawa. iv + 32 pp. Additional copies: For copies of the management plan, or for additional information on species at risk, including COSEWIC Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the SAR Public Registry. Cover illustration: K.L. Yamanaka, Fisheries and Oceans Canada Également disponible en français sous le titre « Plan de gestion visant le sébaste aux yeux jaunes (Sebastes ruberrimus) au Canada [Proposition]» © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2018. All rights reserved. ISBN ISBN to be included by SARA Responsible Agency Catalogue no. Catalogue no. to be included by SARA Responsible Agency Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. Management Plan for the Yelloweye Rockfish [Proposed] 2018 Preface The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of a management plan for species listed as special concern and are required to report on progress five years after the publication of the final document on the Species at Risk Public Registry. -

Ÿþm Icrosoft W
MOODY MARINE LTD Authors: Joe Powers, Geoff Tingley, Susan Hanna, Paul Knapman Public Comment Draft Report for THE GULF OF ALASKA NORTHERN ROCK SOLE TRAWL FISHERY Client: Best Use Coalition Certification Body: Client Contact: Moody Marine Ltd John Gauvin Moody International Certification Best Use Coalition 28 Fleming Drive c/o Groundfish Forum Halifax 4241 21st Ave West Nova Scotia Suite 200 Canada Seattle B3P 1A9 Washington, 98199 Tel: +1 902 489 5581 +1 206 213 5270 FN 82050 Northern Rock Sole GOA V3 January 2010 Page 1 CONTENTS SUMMARY .......................................................................................................................................................4 1. INTRODUCTION ...................................................................................................................................6 1.1 THE FISHERY PROPOSED FOR CERTIFICATION .........................................................................................6 1.2 REPORT STRUCTURE AND ASSESSMENT PROCESS .................................................................................7 1.3 INFORMATION SOURCES USED................................................................................................................8 2 GLOSSARY OF ACRONYMS, ABBREVIATIONS AND SOME DEFINITIONS USED IN THE REPORT...................................................................................................................................13 3 BACKGROUND TO THE FISHERY .................................................................................................15 -

INVERTEBRATE SPECIES in the EASTERN BERING SEA By
Effects of areas closed to bottom trawling on fish and invertebrate species in the eastern Bering Sea Item Type Thesis Authors Frazier, Christine Ann Download date 01/10/2021 18:30:05 Link to Item http://hdl.handle.net/11122/5018 e f f e c t s o f a r e a s c l o s e d t o b o t t o m t r a w l in g o n fish a n d INVERTEBRATE SPECIES IN THE EASTERN BERING SEA By Christine Ann Frazier RECOMMENDED: — . /Vj Advisory Committee Chair Program Head / \ \ APPROVED: M--- —— [)\ Dean, School of Fisheries and Ocean Sciences • ~7/ . <-/ / f a Dean of the Graduate Sch6oI EFFECTS OF AREAS CLOSED TO BOTTOM TRAWLING ON FISH AND INVERTEBRATE SPECIES IN THE EASTERN BERING SEA A THESIS Presented to the Faculty of the University of Alaska Fairbanks in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE 6 By Christine Ann Frazier, B.A. Fairbanks, Alaska December 2003 UNIVERSITY OF ALASKA FAIRBANKS ABSTRACT The Bering Sea is a productive ecosystem with some of the most important fisheries in the United States. Constant commercial fishing for groundfish has occurred since the 1960s. The implementation of areas closed to bottom trawling to protect critical habitat for fish or crabs resulted in successful management of these fisheries. The efficacy of these closures on non-target species is unknown. This study determined if differences in abundance, biomass, diversity and evenness of dominant fish and invertebrate species occur among areas open and closed to bottom trawling in the eastern Bering Sea between 1996 and 2000.