Comprehensive Comparison Table (Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Goals and Outcomes – Gametogenesis, Fertilization (Embryology Chapter 1)
Department of Histology and Embryology, Faculty of Medicine in Pilsen, Charles University, Czech Republic; License Creative Commons - http://creativecommons.org/licenses/by-nc-nd/3.0/ Goals and outcomes – Gametogenesis, fertilization (Embryology chapter 1) Be able to: − Define and use: progenesis, gametogenesis, primordial gonocytes, spermatogonia, primary and secondary spermatocytes, spermatids, sperm cells (spermatozoa), oogonia, primary and secondary oocytes, polar bodies, ovarian follicles (primordial, primary, secondary, tertiary), membrane granulosa, cumulus oophorus, follicular antrum, theca folliculi interna and externa, zona pellucida, corona radiata, ovulation, corpus luteum, corpus albicans, follicular atresia, expanded cumulus, luteinizing hormone (LH), follicle-stimulating hormone (FSH), human chorionic gonadotropin (hCG), sperm capacitation, acrosome reaction, cortical reaction and zona reaction, fertilization, zygote, cleavage, implantation, gastrulation, organogenesis, embryo, fetus, cell division, differentiation, morphogenesis, condensation, migration, delamination, apoptosis, induction, genotype, phenotype, epigenetics, ART – assisted reproductive techniques, spermiogram, IVF-ET (in vitro fertilization followed by embryo transfer), GIFT – gamete intrafallopian transfer, ICSI – intracytoplasmatic sperm injection − Draw and label simplified developmental schemes specified in a separate document. − Give examples of epigenetic mechanisms (at least three of them) and explain how these may affect the formation of phenotype. − Give examples of ethical issues in embryology (at least three of them). − Explain how the sperm cells are formed, starting with primordial gonocytes. Compare the nuclear DNA content, numbers of chromosomes, cell shape and size in all stages. − Explain how the Sertoli cells and Leydig cells contribute to spermatogenesis. − List the parameters used for sperm analysis. What are their normal values? − Explain how the mature oocytes differentiate, starting with oogonia. − Explain how the LH and FSH contribute to oogenesis. -

Development of the Endochondral Skeleton
Downloaded from http://cshperspectives.cshlp.org/ on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Development of the Endochondral Skeleton Fanxin Long1,2 and David M. Ornitz2 1Department of Medicine, Washington University School of Medicine, St. Louis, Missouri 63110 2Department of Developmental Biology, Washington University School of Medicine, St. Louis, Missouri 63110 Correspondence: fl[email protected] SUMMARY Much of the mammalian skeleton is composed of bones that originate from cartilage templates through endochondral ossification. Elucidating the mechanisms that control endochondral bone development is critical for understanding human skeletal diseases, injury response, and aging. Mouse genetic studies in the past 15 years have provided unprecedented insights about molecules regulating chondrocyte formation, chondrocyte maturation, and osteoblast differ- entiation, all key processes of endochondral bone development. These include the roles of the secreted proteins IHH, PTHrP, BMPs, WNTs, and FGFs, their receptors, and transcription factors such as SOX9, RUNX2, and OSX, in regulating chondrocyte and osteoblast biology. This review aims to integrate the known functions of extracellular signals and transcription factors that regulate development of the endochondral skeleton. Outline 1 Introduction 5 Osteoblastogenesis 2 Mesenchymal condensation 6 Closing remarks 3 Chondrocyte differentiation References 4 Growth plate development Editors: Patrick P.L. Tam, W. James Nelson, and Janet Rossant Additional Perspectives on Mammalian Development available at www.cshperspectives.org Copyright # 2013 Cold Spring Harbor Laboratory Press; all rights reserved; doi: 10.1101/cshperspect.a008334 Cite this article as Cold Spring Harb Perspect Biol 2013;5:a008334 1 Downloaded from http://cshperspectives.cshlp.org/ on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press F. -

Germ Cells …… Do Not Appear …… Until the Sixth Week of Development
Reproductive System Session 1 Origin of the Sexes Lecture 1 Development of Male and Female Reproductive System 1 The genital system LANGMAN”S Medical Embryology Indifferent Embryo • Between week 1 and 6, female and male embryos are phenotypically indistinguishable, even though the genotype (XX or XY) of the embryo is established at fertilization. • By week 12, some female and male characteristics of the external genitalia can be recognized. • By week 20, phenotypic differentiation is complete. 4 Indifferent Embryo • The indifferent gonads develop in a longitudinal elevation or ridge of intermediate mesoderm called the urogenital ridge ❑ Initially…. gonads (as a pair of longitudinal ridges, the genital or gonadal ridges). ❑ Epithelium + Mesenchyme. ❑ Germ cells …… do not appear …… until the sixth week of development. • Primordial germ cells arise from the lining cells in the wall of the yolk sac at weeks 3-4. • At week 4-6, primordial germ cells migrate into the indifferent gonad. ➢ Male germ cells will colonise the medullary region and the cortex region will atrophy. ➢ Female germ cells will colonise the cortex of the primordial gonad so the medullary cords do not develop. 5 6 The genital system 7 8 • Phenotypic differentiation is determined by the SRY gene (sex determining region on Y). • which is located on the short arm of the Y chromosome. The Sry gene encodes for a protein called testes- determining factor (TDF). 1. As the indifferent gonad develops into the testes, Leydig cells and Sertoli cells differentiate to produce Testosterone and Mullerian-inhibiting factor (MIF), respectively. 3. In the presence of TDF, testosterone, and MIF, the indifferent embryo will be directed to a male phenotype. -

Comparative Studies on Gonad Development in the Rat, the Pig and in Cattle J
Proceedings of the Iowa Academy of Science Volume 49 | Annual Issue Article 96 1942 Comparative Studies on Gonad Development in the Rat, the Pig and in Cattle J. D. Thomson State University of Iowa Copyright © Copyright 1942 by the Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Thomson, J. D. (1942) "Comparative Studies on Gonad Development in the Rat, the Pig and in Cattle," Proceedings of the Iowa Academy of Science: Vol. 49: No. 1 , Article 96. Available at: https://scholarworks.uni.edu/pias/vol49/iss1/96 This Research is brought to you for free and open access by UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Thomson: Comparative Studies on Gonad Development in the Rat, the Pig and COMPARATIVE STUDIES ON GONAD DEVELOPMENT IN THE RAT, THE PIG AND IN CATTLP1 J J. D. THOMSON INTRODUCTIO~ The relatively clear and simple developmental pattern of the frog gonad (Witschi 1914, 1924, 1929) makes it a good basic type with which to compare the sex glands of higher vertebrates. The frog gonad, before sex differentiation, consists of a germin al epithelium (cortex) containing germ cells and follicle cells, of a mesenchyme-filled primary gonad cavity (this mesenchyme later forming the primary albuginea), and of a series of rete cords (of mesonephric blastema origin) entering through the hilum and pro jecting into the primary gonad cavity. The rete cords constitute the primitive medulla. -
Upper Limb Development
Upper Limb Development Alphonsus Chong Department of Hand and Reconstructive Microsurgery National University Hospital Why bother? Most congenital limb anomalies are due to: Disorders of embryogenesis or Problems during fetal development Some terminology Embryogenesis 0-8 weeks – new organ systems appear Fetal period Appearance of primary ossification center in humerus Differentiation, maturation and enlargement of existing organs Limb Development Limb Patterning Tissue Differentiation Why is it an arm and not Skeletal a leg? Joint Vascular Nerve Muscle and Tendon Positional Information and Axes of the upper limb Limb Bud in E3 Chick Embryo Limb bud (lateral plate) Loose mesenchymal cells from lateral plate mesoderm Ectodermal epithelial cells Migrating cells Somites --> Muscle Nerves Vasculature Limb Bud Development Limb bud Ectoderm and mesenchyme Not fully differentiated yet but all ingredients there If transplanted ectopic limb Limb Bud Regions AER Progress zone Zone of polarizing activity AER – Proximal to Distal formation Zone of Polarizing Actvity – AP development Morphogen Gradient Model Dorsal / ventral patterning less well understood Separation of Digits Apoptosis (Programmed cell death) of interdigital mesenchyme BMPs important Starts post-axial to pre-axial Mesoderm specifies amount of apoptosis How does this relate to pathogensis? Picture from Greene Learning Points UE development occurs early in embryogenesis – most risk of development congenital anomalies Pattern of limb development follows a body plan Digit formation is by apoptosis Thank You Further Reading Principles of Development 3rd Ed by Lewis Wolpert. Oxford University Press Growing Hand. Amit Gupta and Louisville Group. -

A 29-Year-Old Man with Acute Onset Blurry Vision, Weakness, and Gait Abnormality
Journal of the Louisiana State Medical Society CLINICAL CASE OF THE MONTH A 29-Year-Old Man With Acute Onset Blurry Vision, Weakness, and Gait Abnormality Deepu Thoppil, MD; Murtuza J. Ali, MD; Neeraj Jain, MD; Sanjay Kamboj, MD; Pramilla Subramaniam, MD; and Fred A. Lopez, MD (Section Editor) A 29-year-old man, with no significant past medical history, was in his usual state of health until the afternoon of admission. The patient was seated at work eating lunch when he suddenly noticed that his vision became blurry. He covered his right eye and had no visual difficulty but noted blurry vision upon covering his left eye. At this point, the patient tried to stand up, but had difficulty walking and noticed he was “falling toward his left.” Facial asymmetry when smiling was also appreciated. The patient denied any alteration in mental status, confusion, antecedent or current headaches, aura, chest pains, or shortness of breath. He was not taking any prescribed medications and had no known allergies. The patient denied any prior hospitalization or surgery. He denied use of tobacco, alcohol, or illicit drugs, and worked as a maintenance worker in a hotel. His family history is remarkable for his father who died of pancreatic cancer in his 50s and his mother who died of an unknown heart condition in her late 40s. Vital signs on presentation to the emergency department included temperature of 97.6oF; respiratory rate of 18 per minute; pulse of 68 per minute; blood pressure of 124/84 mmHg; pulse oximetry of 99% on ambient air. -
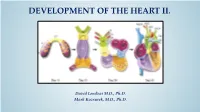
Development of Right Ventricle
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left. -

Journal of Feline Medicine and Surgery
Journal of Feline Medicine and Surgery http://jfm.sagepub.com/ Partial urorectal septum malformation sequence in a kitten with disorder of sexual development Brice S Reynolds, Amélie Pain, Patricia Meynaud-Collard, Joanna Nowacka-Woszuk, Izabela Szczerbal, Marek Switonski and Sylvie Chastant-Maillard Journal of Feline Medicine and Surgery published online 9 April 2014 DOI: 10.1177/1098612X14529958 The online version of this article can be found at: http://jfm.sagepub.com/content/early/2014/04/09/1098612X14529958 Disclaimer The Journal of Feline Medicine and Surgery is an international journal and authors may discuss products and formulations that are not available or licensed in the individual reader's own country. Furthermore, drugs may be mentioned that are licensed for human use, and not for veterinary use. Readers need to bear this in mind and be aware of the prescribing laws pertaining to their own country. Likewise, in relation to advertising material, it is the responsibility of the reader to check that the product is authorised for use in their own country. The authors, editors, owners and publishers do not accept any responsibility for any loss or damage arising from actions or decisions based on information contained in this publication; ultimate responsibility for the treatment of animals and interpretation of published materials lies with the veterinary practitioner. The opinions expressed are those of the authors and the inclusion in this publication of material relating to a particular product, method or technique does not -

Homeobox Genes D11–D13 and A13 Control Mouse Autopod Cortical
Research article Homeobox genes d11–d13 and a13 control mouse autopod cortical bone and joint formation Pablo Villavicencio-Lorini,1,2 Pia Kuss,1,2 Julia Friedrich,1,2 Julia Haupt,1,2 Muhammed Farooq,3 Seval Türkmen,2 Denis Duboule,4 Jochen Hecht,1,5 and Stefan Mundlos1,2,5 1Max Planck Institute for Molecular Genetics, Berlin, Germany. 2Institute for Medical Genetics, Charité, Universitätsmedizin Berlin, Berlin, Germany. 3Human Molecular Genetics Laboratory, National Institute for Biotechnology & Genetic Engineering (NIBGE), Faisalabad, Pakistan. 4National Research Centre Frontiers in Genetics, Department of Zoology and Animal Biology, University of Geneva, Geneva, Switzerland. 5Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité, Universitätsmedizin Berlin, Berlin, Germany. The molecular mechanisms that govern bone and joint formation are complex, involving an integrated network of signaling pathways and gene regulators. We investigated the role of Hox genes, which are known to specify individual segments of the skeleton, in the formation of autopod limb bones (i.e., the hands and feet) using the mouse mutant synpolydactyly homolog (spdh), which encodes a polyalanine expansion in Hoxd13. We found that no cortical bone was formed in the autopod in spdh/spdh mice; instead, these bones underwent trabecular ossification after birth. Spdh/spdh metacarpals acquired an ovoid shape and developed ectopic joints, indicating a loss of long bone characteristics and thus a transformation of metacarpals into carpal bones. The perichon- drium of spdh/spdh mice showed abnormal morphology and decreased expression of Runt-related transcription factor 2 (Runx2), which was identified as a direct Hoxd13 transcriptional target. Hoxd11–/–Hoxd12–/–Hoxd13–/– tri- ple-knockout mice and Hoxd13–/–Hoxa13+/– mice exhibited similar but less severe defects, suggesting that these Hox genes have similar and complementary functions and that the spdh allele acts as a dominant negative. -

Urinary System Intermediate Mesoderm
Urinary System Intermediate mesoderm lateral mesoderm: somite ectoderm neural NOTE: Intermediate mesoderm splanchnic groove somatic is situated between somites and lateral mesoderm (somatic and splanchnic mesoderm bordering the coelom). All mesoderm is derived from the primary mesen- intermediate mesoderm endoderm chyme that migrated through the notochord coelom (becomes urogenital ridge) primitive streak. Intermediate mesoderm (plus adjacent mesothelium lining the coelom) forms a urogenital ridge, which consists of a laterally-positioned nephrogenic cord (that forms kidneys & ureter) and a medially-positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Thus urinary & genital systems have a common embryonic origin; also, they share common ducts. NOTE: Urine production essentially requires an increased capillary surface area (glomeruli), epithelial tubules to collect plasma filtrate and extract desirable constituents, and a duct system to convey urine away from the body. Kidneys Bilateraly, three kid- mesonephric duct neys develop from the neph- metanephros pronephros rogenic cord. They develop mesonephric tubules chronologically in cranial- mesonephros caudal sequence, and are designated pro—, meso—, Nephrogenic Cord (left) and meta—, respectively. cloaca The pronephros and mesonephros develop similarly: the nephrogenic cord undergoes seg- mentation, segments become tubules, tubules drain into a duct & eventually tubules disintegrate. spinal ganglion 1] Pronephros—consists of (7-8) primitive tubules and a pronephric duct that grows caudally and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the neural tube mesonephric duct. (The pronephros is not functional, somite except in sheep.) notochord mesonephric NOTE tubule The mesonephros is the functional kidney for fish and am- aorta phibians. The metanephros is the functional kidney body of reptiles, birds, & mammals. -

Comparative Studies on Gonad Development in the Rat, the Pig and in Cattle
Proceedings of the Iowa Academy of Science Volume 49 Annual Issue Article 96 1942 Comparative Studies on Gonad Development in the Rat, the Pig and in Cattle J. D. Thomson State University of Iowa Let us know how access to this document benefits ouy Copyright ©1942 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Thomson, J. D. (1942) "Comparative Studies on Gonad Development in the Rat, the Pig and in Cattle," Proceedings of the Iowa Academy of Science, 49(1), 475-501. Available at: https://scholarworks.uni.edu/pias/vol49/iss1/96 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Thomson: Comparative Studies on Gonad Development in the Rat, the Pig and COMPARATIVE STUDIES ON GONAD DEVELOPMENT IN THE RAT, THE PIG AND IN CATTLP1 J J. D. THOMSON INTRODUCTIO~ The relatively clear and simple developmental pattern of the frog gonad (Witschi 1914, 1924, 1929) makes it a good basic type with which to compare the sex glands of higher vertebrates. The frog gonad, before sex differentiation, consists of a germin al epithelium (cortex) containing germ cells and follicle cells, of a mesenchyme-filled primary gonad cavity (this mesenchyme later forming the primary albuginea), and of a series of rete cords (of mesonephric blastema origin) entering through the hilum and pro jecting into the primary gonad cavity.