A Cylindrical Triode Ultrahigh Vacuum Ionization Gauge with a Carbon Nanotube Cathode
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE HOT-CATHODE DISCHARGE in HELIUM by Michael A. Gusinow A
The hot-cathode discharge in helium Item Type text; Thesis-Reproduction (electronic) Authors Gusinow, Michael Allen, 1939- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 02/10/2021 14:07:46 Link to Item http://hdl.handle.net/10150/319504 THE HOT-CATHODE DISCHARGE IN HELIUM by Michael A. Gusinow A Thesis Submitted to the Faculty of the DEPARTMENT OF ELECTRICAL ENGINEERING In Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 1963 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfillment of re quirements for an advanced degree at The University of Arizona and is deposited in the University Library to be made available to bor rowers under rules of the Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowledgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in their judgment the proposed use of the material is in the interests of scholarship. In all other instances, however, permission must be obtained from the author. SIGNED APPROVAL BY THESIS DIRECTOR This thesis has been approved on the date shown below: / Date Associate Professor of Electrical Engineering ii ACKNOWLEDGMENTS The author wishes to express his appreciation to Dr. -

Series 600 Hot Cathode Bayard-Alpert Ionization Vacuum Gauges
InstruTech® Series 600 Hot Cathode Bayard-Alpert Ionization Vacuum Gauges BA600 Mini IG: Electron Bombardment (EB) degas design 1 x 10-9 to 5 x 10-2 Torr Measurement Range BA601 UHV Nude: Electron Bombardment (EB) degas design 2 x 10-11 to 1 x 10-3 Torr Measurement Range 2 BA602/BA603: Resistive degas (I R) design BA600 BA601 -10 -3 4 x 10 to 1 x 10 Torr Measurement Range Wide range of emission currents (100 μA to 10 mA) All 4 models can be degassed using electron bombardment, BA602/BA603 can also be degassed using resistive degas (I2R) BA602 BA603 Description The hot cathode Bayard-Alpert ionization vacuum gauge (IG) operates by ionizing the Collector gas inside the gauge and then measuring the number of ions generated. The ions are Filament 1 Grid then collected giving a measurement of the density or pressure of the gas inside the Filament 2 transducer. The various electrodes used in the transducer design are a collector surrounded by a circular grid with one or two filaments outside the grid. An electric current is passed through the filament to cause the filament temperature to increase. As the filament temperature is increased, electrons are emitted from the filament surface. The bias voltage between the filament and the grid will accelerate the electrons toward the grid. Most electrons will pass through the grid volume and exit the other side of the grid and then be drawn back into the grid for another traversal through the grid volume. Eventually, most electrons will impact the grid surface generating a current between the filament and the grid which is referred to as the emission current. -

Valve Types and Char
‘Technical Shorts’ by Gerry O’Hara, VE7GUH/G8GUH ‘Technical Shorts’ is a series of (fairly) short articles prepared for the Eddystone User Group (EUG) website, each focussing on a technical issue of relevance in repairing, restoring or using Eddystone valve radios. However, much of the content is also applicable to non-Eddystone valve receivers. The articles are the author’s personal opinion, based on his experience and are meant to be of interest or help to the novice or hobbyist – they are not meant to be a definitive or exhaustive treatise on the topic under discussion…. References are provided for those wishing to explore the subjects discussed in more depth. The author encourages feedback and discussion on any topic covered through the EUG forum. Valve Types and Characteristics Introduction The Technical Short on ‘Valve Lore’ deals with using valves in receivers in general terms – their evolution through the years, types and general application in Eddystone receivers, as well as tips on sourcing valves and testing them. The Technical Shorts on ‘Eddystone Circuit Elements’ and ‘Receiver Front-Ends’ take a closer look at how valves are selected and used in particular circuits within Eddystone valve receivers. In preparing these articles, it occurred to me that an insight into some of the basics underlying the selection of a particular valve for an application in a receiver would be useful. In order to do this, some consideration of valve ‘fundamentals’ is necessary and how these influence their application and use in receivers. So here I deal mainly with the basics of valve construction and design and then their electrical parameters and important operating characteristics. -

HPS Series 919 Hot Cathode Ionization High Vacuum Sensor
HPS® Products Series 919 Hot Cathode Ionization High Vacuum Sensor System idealvac.com (505)872-0037 OPERATION AND idealvac.com MAINTENANCE MANUAL HPS® Products Series 919 Hot Cathode Ionization High Vacuum Sensor System May 1997 Part # 109190098 Rev. B1 Hot Cathode Ionization High Vacuum Senor System Part # 919-A-XXX-XX __ __ __ - __ __ Serial # __ __ __ __ __ __ Please fill in these numbers and have them readily available when calling for service or additional information. (The part number can be found on your packing slip, and the serial number is located on the rear panel of the Controller.) For more information or literature, contact: MKS Instruments,HPS® Division Inc. 5330 Sterling Drive Boulder, CO 80301 USA Phone: 303-449-9861 800-345-1967 FAX: 303-442-6880 © 1997 by MKS Instruments,HPS® Division Inc. All rights reserved. Inconel is a registered trademark of Inco Alloys International, Inc. SensaVac is a trademark of MKS Instruments, Inc. Hot Cathode Ionization High Vacuum Senor System Table of Contents Table of Contents Package Contents ..................................................................... 1 Symbols Used in this Manual .................................................... 2 Symboles utilisés dans ce manuel .......................................................... 3 In dieser Betriebsanleitung vorkommende Symbole ................................ 4 Símbolos Usados en el Manual ............................................................... 5 Symbols Found on the Unit ...................................................... -

Unusual Tubes
Unusual Tubes Tom Duncan, KG4CUY March 8, 2019 Tubes On Hand GAS-FILLED HIGH-VACUUM • Neon Lamp (NE-51) • Photomultiplier • Cold-cathode Voltage (931A) Regulator (0B2) • Magic Eye (1629) • Hot-cathode Thyratron • Low-voltage (12DY8) (884) • Space Charge (12K5) 2 Timeline of Related Events 1876, 1902 William Crookes Cathode Rays, Glow Discharge 1887 [1921] Hertz, Einstein Photoelectric Effect 1897 [1906] J. J. Thomson Electron identified 1920 Daniel Moore (GE) Voltage Regulator 1923 Joseph Slepian Secondary Emission (Westinghouse) 1928 Albert Hull, Irving Thyratron Langmuir (GE) [1928] Owen Richardson Thermionic Emission 1936 Vladimir Zworykin Photomultiplier (RCA) 1937 Allen DuMont Magic Eye 3 Neon Bulbs • Based on glow-discharge (coronal discharge) effect noticed by William Crookes around 1902. • Exhibit a negative incremental resistance over part of the operating range. • Light-sensitive: photo-ionization causes the ionization voltage to decrease with illumination (not generally a desirable characteristic). • Used as indicators , voltage regulators, relaxation oscillators , and the larger ones for illumination . 4 Neon Lamp/VR Tube Curves 80 Normal Abnormal Glow Glow 70 60 Townsend Discharge 50 Negative Resistance 40 Region 30 Volts across Device across Volts 20 10 Conduction Destroys Lamp Destroys Arc Conduction Arc Chart details (coronal) Glow depend on -5 element 10 -20 10 -15 10 -10 10 1 geometry and Current through Device (A) gas mixture. 5 Cold-Cathode Voltage Regulator Tubes • Very similar to neon bulbs: attention paid to increasing current-carrying capability and ensuring a constant forward voltage. • Gas sometimes includes radio-isotopes to reduce sensitivity to photo-ionization. • Developed at General Electric Research Labs by Daniel Moore around 1920. -

Bibliography and Index on Vacuum And
NATL INST OF STANDARDS & TECH R.I.C. All 100988737 /NBS monograph QC100 .U556 W5;SUPP1;1967 C.I NBS-PUB-C NBS PUBLICATIONS NBS MONOGRAPH 35—Supplement 1 Bibliography and Index on Vacuum and Low Pressure IVIeasurement January 1960 to December 1965 U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS — THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards^ provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation's scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coordinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Physics—Physical Chemistry—Radiation Physics—Laboratory Astrophysics-—Radio Standards Laboratory,^ which includes Radio Standards Physics and Radio Standards Engineering- -Office of Standard Refer- ence Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of ma- terials. Beyond its direct interest to the Nation's scientists and engineers, this Institute yields services which are essential to the advancement of technology in industry and commerce. -

Instruction Manual
Instruction Manual CAT. No. B10-7260-W0 Crookes Tube with Electrode Deflecting Plate IE-A Page 1 (Hot Cathode Type) CAT. No. B10-2483-W0 Crookes Tube Power Supply C-HC (Hot Cathode Type) Page 6 Sept. 2020 Printed in Japan Cat. No. B10-7260-W0 Crookes Tube with Electrode Deflecting Plate (Hot Cathode Type) Instruction Manual 1. Purpose This tube is designed for the experimental observation of deflection phenomena caused by electric and magnetic fields of cathode ray. It may also be used to demonstrate the principles of a cathode ray tube (CRT). 2. Construction and Principles An electron gun consisting of a cylindrical plate, a cathode, and a heater is attached along with an electrode deflecting plate inside a CRT-type Crookes tube filled with argon gas. An electron is propelled from the cathode into the cylindrical plate. Voltage applied on the plate accelerates the speed of electron up to a specified velocity by the time the electron is projected through the hole of the plate. The emission resulting from the collision of the electron with the argon gas contained in the tube allows its path to be clearly visible. Heater Argon gas Electron gun Cathode & Anode Electrode deflecting plate Base unit Electrode deflecting plate 2 3. Operation with Crookes Tube Power Supply (B10-2483-W0) ● Connection ◎ Connect the terminals on the black base plate attached to the Crookes tube holder marked with the green terminals on the power supply rear panel (for filament). Connect the terminals on the plate marked (Deflection Plate Upper/Lower) with blue terminals of the power supply (marked “Deflection), and connect the Anode terminal and Cathode terminal on the plate with the red terminal (anode) and the black terminal (cathode) of the power supply, respectively. -
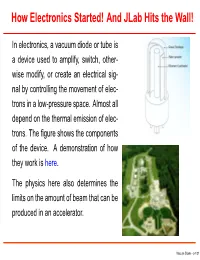
How Electronics Started! and Jlab Hits the Wall!
How Electronics Started! And JLab Hits the Wall! In electronics, a vacuum diode or tube is a device used to amplify, switch, other- wise modify, or create an electrical sig- nal by controlling the movement of elec- trons in a low-pressure space. Almost all depend on the thermal emission of elec- trons. The figure shows the components of the device. A demonstration of how they work is here. The physics here also determines the limits on the amount of beam that can be produced in an accelerator. Vacuum Diode – p.1/21 The Child-Langmuir Law In a vacuum diode, electrons are boiled off a hot cathode at zero potential and accelerated across a gap to the anode at a positive potential V0. The cloud of moving electrons within the gap (called the space charge) builds up and reduces the field at the cathode surface to zero. From then on a steady current flows between the plates. Suppose two plates are large relative to the separation (A>>d2 in the figure) so that edge effects can be ignored and V , ρ, and the electron speed v are functions of only x. d Electron A x Cathode (V=0) Anode(V=V0 ) Vacuum Diode – p.2/21 The Child-Langmuir Law 1. What is Poisson’s equation for the region between the plates? 2. Assuming the electrons start from rest at the cathode, what is their speed at point x? 3. In the steady state I, the current, is independent of x. How are ρ and v related? 4. Now generate a differential equation for V by eliminating ρ and v and solve this equation for V as a function of x, V0, and d. -

A Micromachined Vacuum Triode Using a Carbon Nanotube Cold Cathode Chris Bower, Diego Shalóm, Wei Zhu, Daniel López, Greg P
1478 IEEE TRANSACTIONS ON ELECTRON DEVICES, VOL. 49, NO. 8, AUGUST 2002 A Micromachined Vacuum Triode Using a Carbon Nanotube Cold Cathode Chris Bower, Diego Shalóm, Wei Zhu, Daniel López, Greg P. Kochanski, Peter L. Gammel, Member, IEEE, and Sungho Jin Abstract—A fully integrated on-chip vacuum microtriode using vacuum tube amplifieirs can be compact and efficient for many carbon nanotubes as field emitters was constructed laterally on a high power and high frequency applications. This is in part due silicon surface using microelectromechanical systems (MEMS) de- to the fundamental speed of electrons in vacuum being about sign and fabrication principles. Each electrode in the triode was made of a hinged polycrystalline silicon panel that could be ro- three orders of magnitude faster (with correspondingly less en- tated and locked into an upright position. The device was operated ergy loss) than in solids. They are also radiation hard and can at a current density as high as 16 A/cmP. Although the transcon- be operated over a wider temperature range. Therefore, vacuum ductance was measured only at 1.3 S, the dc output power deliv- tubes remain the amplifier of choice for radar, electronic war- ered at the anode was almost 40 more than the power lost at the fare and space-based communications. grid electrode. The technique combines high-performance nano- materials with mature solid-state fabrication technology to pro- The use of cold cathodes in vacuum devices can potentially duce miniaturized power-amplifying vacuum devices in an on-chip bring together the best features of both vacuum tubes (e.g., high form, which could potentially offer a route of integrating vacuum power) and solid state power transistors (e.g., long lifetime and and solid-state electronics and open up new applications for “old- miniaturization). -

RCA Receiving Tubes Are Classified in the Following Chart According to Func- Tion and Filament Or Heater Voltage
PRICE FIFTY CENTS RADIO CORPORATION of AMERICA TW/BE DEPARTMENT HARRISON. N. J. CONTENTS PAGE ELECTRONS, ELECTRODES, AND ELECTRON TUBES . 3 Electrons, Cathodes, Generic Tube Types, Diodes, Triodes, Pentodes, Beam Power Tubes, Multi -Electrode and Multi -Unit Types, Kinescopes ELECTRON TUBE CHARACTERISTICS 11 ELECTRON TUBE APPLICATIONS 13 Amplification, Rectification, Detection, Automatic Volume Control, Tuning Indication with Electron -Ray Tubes, Oscillation, Frequency Conversion, Automatic Frequency Control ELECTRON TUBE INSTALLATION 47 Filament and Heater Supply, Heater -to-Cathode Connection, Plate Voltage Supply, Grid Voltage Supply, Screen Voltage Supply, Shield- ing, Dress of Circuit Leads, Filters, Output -Coupling Devices,, High - Voltage Considerations for Kinescopes, Kinescope Safety Considerations INTERPRETATION OF TUBE DATA 58 RECEIVING TUBE CLASSIFICATION CHART 62 TUBE TYPES- Technical Data 65 ELECTRON TUBE TESTING 242 RESISTANCE -COUPLED AMPLIFIERS 246 CIRCUITS 263 OUTLINES 284 INDEX 301 RECENTLY ADDED TUBE TYPES 305 READING LIST 336 Key to Socket Connection Diagrams Bottom Views BC =Base Sleeve G = Grid K = Cathode BS =Base Shell H = Heater NC =No Connection C = External Conduc- HL = Heater Tap for P =Plate (Anode) tive Coating Panel Lamp RC = Ray- Control DJ = Deflecting Elec- Hive = Heater Mid - Electrode trode Tap S =Shell ES = External Shield IC = Internal Connec- F = Filament tion- TA = Target FM = Filament Mid- Do Not Use U =Unit Tap IS =Internal Shield = Gas -Type Tube Alphabetical Subscripts B.D,HP,HX,P, and T indicate, respectively, beam unit, diode unit, heptode unit, hexode unit, pentode unit, and triode unit in multi-unit types. Devices and arrangements shown or described herein may use patents of RCA or others. Information contained herein is furnished without respon- sibility by RCA for its use and without prejudice to RCA's patent rights. -

Perrin Tube D 1000650
3B SCIENTIFIC® PHYSICS Perrin Tube D 1000650 Instruction sheet 10/15 ALF 1 4mm sockets connected to cathode filament and heating 2 Heater filament 3 4mm plug for connecting anode 4 Boss 5 Fluorescent screen 6 Faraday cage - 7 4mm plug connected to Far- aday cage 1 2 3 4 5 6 7 1. Safety instructions 2. Description Hot cathode tubes are thin-walled, highly evac- The Perrin tube serves to demonstrate the negative uated glass tubes. Treat them carefully as there polarity of electrons and to estimate the specific is a risk of implosion. electron charge e/m by magnetic deflection into a Do not subject the tube to mechanical Faraday cage, which is connected to an electro- stresses. scope. It is also possible to investigate the deflec- tion of electrons in two perpendicular magnetic Do not subject the connection leads to any alternating fields and to demonstrate the effects, for tension. example by generating Lissajous figures. The tube may only be used with tube holder D The Perrin tube is a highly evacuated tube with (1008507). an electron gun, consisting of a pure tungsten If voltage or current is too high or the cathode is heater filament and a cylindrical anode contained at the wrong temperature, it can lead to the tube in a clear glass bulb, partly coated with a fluores- becoming destroyed. cent screen. The electrons emitted by the elec- Do not exceed the stated operating parameters. tron gun form a narrow circular beam that can be seen as a spot on the fluorescent screen. A glass Only change circuit with power supply tube with a Faraday cage is set on the glass bulb equipment switched off. -

How Vacuum Tubes Work
How Vacuum Tubes Work http://www.vacuumtubes.net/How_Vacuum_Tubes_Work.htm This Page is a courtesy of www.vacuumtubes.net by Eric Barbour [The Basics] - [Inside a Tube] - [Why are Tubes Still Used] - [Using Tubes] The BASICS Back in 1904, British scientist John Ambrose Fleming first showed his device to convert an alternating current signal into direct current. The "Fleming diode" was based on an effect that Thomas Edison had first discovered in 1880, and had not put to useful work at the time. This diode essentially consisted of an incandescent light bulb with an extra electrode inside. When the bulb's filament is heated white-hot, electrons are boiled off its surface and into the vacuum inside the bulb. If the extra electrode (also called an "plate" or "anode") is made more positive than the hot filament, a direct current flows through the vacuum. And since the extra electrode is cold and the filament is hot, this current can only flow from the filament to the electrode, not the other way. So, AC signals can be converted into DC. Fleming's diode was first used as a sensitive detector of the weak signals produced by the new wireless telegraph. Later (and to this day), the diode vacuum tube was used to convert AC into DC in power supplies for electronic equipment. Many other inventors tried to improve the Fleming diode, most without success. The only one who succeeded was New York inventor Lee de Forest. In 1907 he patented a bulb with the same contents as the Fleming diode, except for an added electrode.