Differential Volume Regulation and Calcium Signaling in Two Ciliary Body Cell Types Is Subserved by TRPV4 Channels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Therapeutic Effect of Agmatine on Neurological Disease: Focus on Ion Channels and Receptors
Neurochemical Research (2019) 44:735–750 https://doi.org/10.1007/s11064-018-02712-1 REVIEW PAPER Therapeutic Effect of Agmatine on Neurological Disease: Focus on Ion Channels and Receptors Sumit Barua1 · Jong Youl Kim1 · Jae Young Kim1 · Jae Hwan Kim4 · Jong Eun Lee1,2,3 Received: 15 October 2018 / Revised: 19 December 2018 / Accepted: 24 December 2018 / Published online: 4 January 2019 © Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract The central nervous system (CNS) is the most injury-prone part of the mammalian body. Any acute or chronic, central or peripheral neurological disorder is related to abnormal biochemical and electrical signals in the brain cells. As a result, ion channels and receptors that are abundant in the nervous system and control the electrical and biochemical environment of the CNS play a vital role in neurological disease. The N-methyl-D-aspartate receptor, 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid receptor, kainate receptor, acetylcholine receptor, serotonin receptor, α2-adrenoreceptor, and acid-sensing ion channels are among the major channels and receptors known to be key components of pathophysiological events in the CNS. The primary amine agmatine, a neuromodulator synthesized in the brain by decarboxylation of L-arginine, can regu- late ion channel cascades and receptors that are related to the major CNS disorders. In our previous studies, we established that agmatine was related to the regulation of cell differentiation, nitric oxide synthesis, and murine brain endothelial cell migration, relief of chronic pain, cerebral edema, and apoptotic cell death in experimental CNS disorders. -

Agmatine and Agmatine Analogs in the Treatment of Epilepsy, Seizure, and Electroconvulsive Disorders Peter A
University of Kentucky UKnowledge Pharmaceutical Sciences Faculty Patents Pharmaceutical Sciences 10-19-2010 Agmatine and Agmatine Analogs in the Treatment of Epilepsy, Seizure, and Electroconvulsive Disorders Peter A. Crooks University of Kentucky, [email protected] Aimee K. Bence David R. Worthen Right click to open a feedback form in a new tab to let us know how this document benefits oy u. Follow this and additional works at: https://uknowledge.uky.edu/ps_patents Part of the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Crooks, Peter A.; Bence, Aimee K.; and Worthen, David R., "Agmatine and Agmatine Analogs in the Treatment of Epilepsy, Seizure, and Electroconvulsive Disorders" (2010). Pharmaceutical Sciences Faculty Patents. 47. https://uknowledge.uky.edu/ps_patents/47 This Patent is brought to you for free and open access by the Pharmaceutical Sciences at UKnowledge. It has been accepted for inclusion in Pharmaceutical Sciences Faculty Patents by an authorized administrator of UKnowledge. For more information, please contact [email protected]. US007816407B2 (12) United States Patent (10) Patent N0.: US 7,816,407 B2 Crooks et al. (45) Date of Patent: Oct. 19, 2010 (54) AGMATINE AND AGMATINE ANALOGS IN The Merck Index, Merck Research Laboratories Division of Merck & THE TREATMENT OF EPILEPSY, SEIZURE, Co., Inc. 1996, p. 35. AND ELECTROCONVULSIVE DISORDERS James O. McNamara, “Drugs Effective in Therapy of the Epilepsies”, Goodman & Gilman’s The Pharmacological Basis of Therapeutics, (75) Inventors: Peter A. Crooks, Lexington, KY (US); Ninth Edition, Chapter 20, pp. 461-486, 1996. Aimee K. Bence, Lexington, KY (US); I. Tayfun Uzbay et al., “Effects of agmatine on ethanol Withdrawal David R. -

Figure S1. Heat Map of R (Pearson's Correlation Coefficient)
Figure S1. Heat map of r (Pearson’s correlation coefficient) value among different samples including replicates. The color represented the r value. Figure S2. Distributions of accumulation profiles of lipids, nucleotides, and vitamins detected by widely-targeted UPLC-MC during four fruit developmental stages. The colors indicate the proportional content of each identified metabolites as determined by the average peak response area with R scale normalization. PS1, 2, 3, and 4 represents fruit samples collected at 27, 84, 125, 165 Days After Anthesis (DAA), respectively. Three independent replicates were performed for each stages. Figure S3. Differential metabolites of PS2 vs PS1 group in flavonoid biosynthesis pathway. Figure S4. Differential metabolites of PS2 vs PS1 group in phenylpropanoid biosynthesis pathway. Figure S5. Differential metabolites of PS3 vs PS2 group in flavonoid biosynthesis pathway. Figure S6. Differential metabolites of PS3 vs PS2 group in phenylpropanoid biosynthesis pathway. Figure S7. Differential metabolites of PS4 vs PS3 group in biosynthesis of phenylpropanoids pathway. Figure S8. Differential metabolites of PS2 vs PS1 group in flavonoid biosynthesis pathway and phenylpropanoid biosynthesis pathway combined with RNA-seq results. Table S1. A total of 462 detected metabolites in this study and their peak response areas along the developmental stages of apple fruit. mix0 mix0 mix0 Index Compounds Class PS1a PS1b PS1c PS2a PS2b PS2c PS3a PS3b PS3c PS4a PS4b PS4c ID 1 2 3 Alcohols and 5.25E 7.57E 5.27E 4.24E 5.20E -

Vitamin D Receptor Promotes Healthy Microbial Metabolites
www.nature.com/scientificreports OPEN Vitamin D receptor promotes healthy microbial metabolites and microbiome Ishita Chatterjee1, Rong Lu1, Yongguo Zhang1, Jilei Zhang1, Yang Dai 2, Yinglin Xia1 ✉ & Jun Sun 1 ✉ Microbiota derived metabolites act as chemical messengers that elicit a profound impact on host physiology. Vitamin D receptor (VDR) is a key genetic factor for shaping the host microbiome. However, it remains unclear how microbial metabolites are altered in the absence of VDR. We investigated metabolites from mice with tissue-specifc deletion of VDR in intestinal epithelial cells or myeloid cells. Conditional VDR deletion severely changed metabolites specifcally produced from carbohydrate, protein, lipid, and bile acid metabolism. Eighty-four out of 765 biochemicals were signifcantly altered due to the Vdr status, and 530 signifcant changes were due to the high-fat diet intervention. The impact of diet was more prominent due to loss of VDR as indicated by the diferences in metabolites generated from energy expenditure, tri-carboxylic acid cycle, tocopherol, polyamine metabolism, and bile acids. The efect of HFD was more pronounced in female mice after VDR deletion. Interestingly, the expression levels of farnesoid X receptor in liver and intestine were signifcantly increased after intestinal epithelial VDR deletion and were further increased by the high-fat diet. Our study highlights the gender diferences, tissue specifcity, and potential gut-liver-microbiome axis mediated by VDR that might trigger downstream metabolic disorders. Metabolites are the language between microbiome and host1. To understand how host factors modulate the microbiome and consequently alter molecular and physiological processes, we need to understand the metabo- lome — the collection of interacting metabolites from the microbiome and host. -

Uncovering New Drug Properties in Target-Based Drug-Drug Similarity Networks
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.12.988600; this version posted June 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Uncovering new drug properties in target-based drug-drug similarity networks Lucret¸iaUdrescu1, Paul Bogdan2, Aimee´ Chis¸ 3, Ioan Ovidiu Sˆırbu3,6, Alexandru Topˆırceanu4, Renata-Maria Varut¸˘ 5, and Mihai Udrescu4,6,* 1”Victor Babes¸” University of Medicine and Pharmacy Timis¸oara, Department of Drug Analysis, Timis¸oara 300041, Romania 2University of Southern California, Ming Hsieh Department of Electrical Engineering, Los Angeles, CA 90089-2563, USA 3”Victor Babes¸” University of Medicine and Pharmacy Timis¸oara, Department of Biochemistry, Timis¸oara 300041, Romania 4University Politehnica of Timis¸oara, Department of Computer and Information Technology, Timis¸oara 300223, Romania 5University of Medicine and Pharmacy of Craiova, Faculty of Pharmacy, Craiova 200349, Romania 6Timis¸oara Institute of Complex Systems, Timis¸oara 300044, Romania *[email protected] ABSTRACT Despite recent advances in bioinformatics, systems biology, and machine learning, the accurate prediction of drug properties remains an open problem. Indeed, because the biological environment is a complex system, the traditional approach – based on knowledge about the chemical structures – cannot fully explain the nature of interactions between drugs and biological targets. Consequently, in this paper, we propose an unsupervised machine learning approach that uses the information we know about drug-target interactions to infer drug properties. -

Role of Amino Acid Metabolism in Angiogenesis T ⁎ Roxana E
Vascular Pharmacology 112 (2019) 17–23 Contents lists available at ScienceDirect Vascular Pharmacology journal homepage: www.elsevier.com/locate/vph Review Role of amino acid metabolism in angiogenesis T ⁎ Roxana E. Oberkersch, Massimo M. Santoro Department of Biology, University of Padua, Italy ABSTRACT The role of endothelial metabolism represents a crucial element governing the formation and the differentiation of blood vessels, termed angiogenesis. Besides glycolysis and fatty acid oxidation, endothelial cells rely on specific amino acids to proliferate, migrate, and survive. In this review we focus on the metabolism of those amino acids and the intermediates that hold an established function within angiogenesis and endothelial pa- thophysiology. We also discuss recent work which provides a rationale for specific amino acid-restricted diets and its beneficial effects on vascular tissues, including extending the life span and preventing the development of a variety of diseases. 1. Introduction and produce up to 85% of their ATP through aerobic glycolysis [5]. It has been hypothesized that the use of glycolysis as the main source of The vascular system is a multi-branched network lined by en- ATP holds several advantages. High glycolytic flux can produce faster dothelial cells (ECs) which delivers oxygen and nutrients to the tissues ATP with respect to oxidative metabolism, which represents an ad- in the body [1]. The ability to expand this network is a process called vantage for ECs in a hypoxic microenvironment [6]. The anaerobic angiogenesis. This process is vital in many physiological settings and is metabolism of ECs might save oxygen towards becoming perivascular also implicated in the pathogenesis of several disorders including: mural cells [7]. -

Agmatine Improves Spatial Memory
macolog Khales et al., J Pharma Reports 2016, 1:2 ar ic h a P l f R o e l p a o n r r t s u o J Journal of Pharmacological Reports Research Article Article OpenOpen Access Access Agmatine Improves Spatial Memory Consolidation: The Role of Nitric Oxide Golnaz Yadollahi Khales1, Atefeh Khajeh2 and Maryam Moosavi1,3* 1Shiraz Neuroscience Research Center, Shiraz University of Medical Sciences, Shiraz, Iran 2Students Research Committee, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran 3Nanomedicine and Nanobiology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran Abstract Objective: The existence of agmatine in the hippocampus suggests a role in memory formation. Endogenous agmatine increases after training in the hippocampus, howbeit the effect of exogenous agmatine on memory is not consistent yet. This work was aimed to assess the differential effect of systemic agmatine on spatial memory consolidation and retrieval. Additionally L-NAME was used to assess if nitric oxide is involved in the effect of agmatine. Methods: Male Sprague-Dawley rats (250-350 g) were trained in water maze single training session. After 24 h the memory of animals were examined in a probe trial which was consisted of a trial without platform. To assess the effect of agmatine (40 mg/kg) on memory consolidation it was administered immediately after training and to assess its effect on memory retrieval it was injected 30 min before probe trial. In order to evaluate the involvement of nitric oxide, L-NAME (3 mg/kg) was co-administered with agmatine. Results: Post-training injection (to assess consolidation phase) of agmatine improved the performance of animals in probe test, while its pre-probe administration (to assess retrieval phase) had no effect. -

The Role of Opioid and Nitrergic Systems in Dual Modulation of Date Published Online: 31/08/2020; Seizure Susceptibility
www.als-journal.com/ ISSN 2310-5380/ August 2020 Review Article Advancements in Life Sciences – International Quarterly Journal of Biological Sciences ARTICLE INFO Open Access Date Received: 16/05/2020; Date Revised: 23/08/2020; The role of opioid and nitrergic systems in dual modulation of Date Published Online: 31/08/2020; seizure susceptibility Authors’ Affiliation: 1,3 2,3 1 4 5 1. Department Muhammad Imran Khan , Farid Ullah Shah , Abdul Wahab , Vahid Nikoui *, Ahmad Reza Dehpour * of Pharmacy, Kohat University of Science and Technology, Abstract 26000 Kohat – Pakistan pilepsy is a chronic disorder presented by recurrent episodes of seizures and affect worldwide 2. Department of Biochemistry, Bannu individuals. The underlying mechanism of seizure is still elusive. Hence, there is still a need to determine Medical College, the contribution of various systems in neurobiology and treatment of seizure. Evidence shows that opioid 28100 Bannu, KPK - and nitrergic systems within the brain interact to modulate various physiological and pathological Pakistan E 3. Drug Detoxification conditions including memory, pain, reward, addiction, depression, and seizure. Various studies revealed that Health Welfare Research Center, diverse dose of opioids such as morphine has dual modulation in seizure susceptibility. For instance, it is Bannu, KPK - reported that morphine at lower doses (0.5, 1, and 3 mg/kg) exerts an anticonvulsant effect in experimental Pakistan 4. Razi Drug seizure models, whereas at higher doses (15, 30, and 60 mg/kg) it could exacerbate the seizure. Similarly, Research Center, Iran nitrergic system has also been observed to possess dual effects in modulating the seizure threshold. Therefore, University of Medical Sciences, Tehran - understanding of opioidergic and nitrergic systems interaction in seizure seems important to achieve the Iran successful goal of seizure management. -

Insights Into the Modulation of Dopamine Transporter Function by Amphetamine, Orphenadrine, and Cocaine Binding
ORIGINAL RESEARCH published: 09 June 2015 doi: 10.3389/fneur.2015.00134 Insights into the modulation of dopamine transporter function by amphetamine, orphenadrine, and cocaine binding Mary Hongying Cheng1, Ethan Block2, Feizhuo Hu1,3, Murat Can Cobanoglu1, Alexander Sorkin2 and Ivet Bahar1*† 1 Department of Computational and Systems Biology, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 2 Department of Cell Biology, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 3 Department of Pharmacology and Pharmaceutical Sciences, School of Medicine, Tsinghua University, Beijing, China Edited by: Christopher Surratt, Human dopamine (DA) transporter (hDAT) regulates dopaminergic signaling in the central Duquesne University, USA nervous system by maintaining the synaptic concentration of DA at physiological levels, Reviewed by: Nicola B. Mercuri, upon reuptake of DA into presynaptic terminals. DA translocation involves the co-transport University of Rome Tor Vergata, Italy of two sodium ions and the channeling of a chloride ion, and it is achieved via alternating Harald H. Sitte, access between outward-facing (OF) and inward-facing states of DAT. hDAT is a target for Medical University Vienna, Austria Thomas Stockner, addictive drugs, such as cocaine, amphetamine (AMPH), and therapeutic antidepressants. Medical University of Vienna, Austria Our recent quantitative systems pharmacology study suggested that orphenadrine (ORPH), an (in collaboration with Harald H. Sitte) anticholinergic agent and anti-Parkinson drug, might be repurposable as a DAT drug. Previous *Correspondence: Ivet Bahar, studies have shown that DAT-substrates like AMPH or -blockers like cocaine modulate the Department of Computational and function of DAT in different ways. However, the molecular mechanisms of modulation remained Systems Biology, School of Medicine, elusive due to the lack of structural data on DAT. -

(12) STANDARD PATENT (11) Application No. AU 2012204162 B2 (19) AUSTRALIAN PATENT OFFICE
(12) STANDARD PATENT (11) Application No. AU 2012204162 B2 (19) AUSTRALIAN PATENT OFFICE (54) Title Chemosensory receptor ligand-based therapies (51) International Patent Classification(s) A61K 31/4709 (2006.01) A61P 3/00 (2006.01) A61K 31/155 (2006.01) A61P 3/04 (2006.01) A61K 31/194 (2006.01) A61P 3/10 (2006.01) A61K 31/198 (2006.01) A61P 7/12 (2006.01) A61K 31/20 (2006.01) A61P 19/10 (2006.01) A61K 31/201 (2006.01) A61P 21/00 (2006.01) A61K 31/353 (2006.01) A61P 25/00 (2006.01) A61K 31/575 (2006.01) A61P 25/22 (2006.01) A61K 31/7016 (2006.01) A61P 25/24 (2006.01) A61K 31/7032 (2006.01) A61P 37/00 (2006.01) A61K 31/704 (2006.01) C12N 5/07 (2010.01) A61P 1/00 (2006.01) (21) Application No: 2012204162 (22) Date of Filing: 2012.01.06 (87) WIPO No: W012/094636 (30) Priority Data (31) Number (32) Date (33) Country 61/430,914 2011.01.07 US (43) Publication Date: 2012.07.12 (44) Accepted Journal Date: 2017.04.20 (71) Applicant(s) Elcelyx Therapeutics, Inc. (72) Inventor(s) Baron, Alain D.;Brown, Martin R.;Jones, Christopher R. G.;Beeley, Nigel R. A.;Fineman, Mark S. (74) Agent / Attorney Spruson & Ferguson, L 35 St Martins Tower 31 Market St, Sydney, NSW, 2000, AU (56) Related Art WO 2007/007189 A2 US 2010/0331419 Al WO 2006/086727 A2 (12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date W O 2012/094636 A3 12 July 2012 (12.07.2012) W I PO I P CT (51) International Patent Classification: -

Regulation of Metabolic Products and Gene Expression in Fusarium Asiaticum by Agmatine Addition
Mycotoxin Res (2013) 29:103–111 DOI 10.1007/s12550-013-0158-y ORIGINAL PAPER Regulation of metabolic products and gene expression in Fusarium asiaticum by agmatine addition Tadahiro Suzuki & Young-Kyung Kim & Hifumi Yoshioka & Yumiko Iwahashi Received: 21 October 2012 /Revised: 26 December 2012 /Accepted: 8 January 2013 /Published online: 31 January 2013 # The Author(s) 2013. This article is published with open access at Springerlink.com Abstract The metabolic products resulting from the culti- contamination of grain with trichothecene mycotoxins vation of F. asiaticum in agmatine were identified using (Bottalico and Perrone 2002; Goswami and Kistler 2004). capillary electrophoresis–time of flight mass spectrometry. The F. graminearum species complex consists of at least 13 Glyoxylic acid was detected from fungal cultures grown in phylogenetically distinct species (Yli-Mattila et al. 2009). agmatine, while it was absent in control cells. The abun- These species tend to produce different, strain-specific trichothe- dance of other metabolic products of the glycolytic pathway cenes including nivalenol, deoxynivalenol (DON), 3- also increased because of agmatine; however, there was no acetyldeoxynivalenol (3ADON), and 15-acetyldeoxynivalnenol increase in the amounts of pyruvic acid or metabolites from (15ADON) (Bottalico and Perrone 2002). Geographically, the tricarboxylic acid cycle. Moreover, gene expression among members of the F. graminearum species complex, levels within Fusarium asiaticum exposed to agmatine were 15ADON producers are -
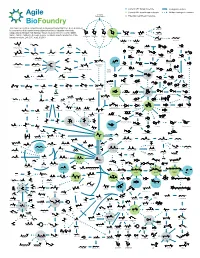
ABF Metabolic Map 15
Current ABF target molecule Biological reaction Current ABF beachhead molecule Multiple biological reactions Biomass Polysaccharides Potential beachhead molecule HO This map represents a compilation of metabolic pathways that have been described OH HO HO Glycolic acid HO HO HO O OH O for conversion of biomass-derived polysaccharides to industrial chemicals. OH OOH O O O OH O OH OH OH HO HO OH OH Adapted by permission from Springer Nature Customer Service Centre GmbH: OH OH OH OH OH Ethylene glycol Nature, Nature Catalysis, A comprehensive metabolic map for production of bio- OH OH OH OH HO OH OH L-Arabinose Galactose Glucose D-Xylose D-xylono-1,4-lactone Xylonic acid OH OH OH based chemicals, Lee, S.Y., et al., © 2019 HO OH OH OH OH HO HO OH HO OH 1,2,4-Butanetriol 1,4-Butanediol O OH OH OH Xylitol D-Arabinitol HO HO O O O HO HO NH2 CoA OH S OH Propanol OH Lactaldehyde 4-Hydroxyhexan-3-one HO OH Malonyl-CoA HO Benzyl alcohol Resveratrol Hydroxytyrosol HO - O O O CoA S O O OH -O O O N HO OH OH O O OH H O O OH HO Benzene O 4-phenylbutyrate O Hippurate Acetol Methylglyoxal Ferulic acid p-Coumaroyl-CoA OH O Propanal 1,2-Propanediol HO 4-Hydroxy-2-oxohexanoic acid p-Hydroxystyrene HO NH2 Benzaldehyde HO OH OH OH Dopamine O OH 1,3-Propanediol O O O O O P - OH O HO O O HO HO HO Glycerone HO OH OH O HO HO 6-phenylhexanoate OH cis, cis-Muconic acid HO O OH O Glyceraldehyde OH HO OH OH HO O O O P - Benzoic acid 3-Phosphate Dihydrobenzenediol Caffeic acid p-Coumaric acid O OH HO 1,3-propanediol 3-Hydroxypropanal HO O HO OH HO NH Glycerol O 2 OH