Thoracic Insufficiency Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Managing a Rib Fracture: a Patient Guide
Managing a Rib Fracture A Patient Guide What is a rib fracture? How is a fractured rib diagnosed? A rib fracture is a break of any of the bones that form the Your doctor will ask questions about your injury and do a rib cage. There may be a single fracture of one or more ribs, physical exam. or a rib may be broken into several pieces. Rib fractures are The doctor may: usually quite painful as the ribs have to move to allow for normal breathing. • Push on your chest to find out where you are hurt. • Watch you breathe and listen to your lungs to make What is a flail chest? sure air is moving in and out normally. When three or more neighboring ribs are fractured in • Listen to your heart. two or more places, a “flail chest” results. This creates an • Check your head, neck, spine, and belly to make sure unstable section of chest wall that moves in the opposite there are no other injuries. direction to the rest of rib cage when you take a breath. • You may need to have an X-ray or other imaging test; For example, when you breathe in your rib cage rises out however, rib fractures do not always show up on X-rays. but the flail chest portion of the rib cage will actually fall in. So you may be treated as though you have a fractured This limits your ability to take effective deep breaths. rib even if an X-ray doesn’t show any broken bones. -

Anesthesia for Trauma
Anesthesia for Trauma Maribeth Massie, CRNA, MS Staff Nurse Anesthetist, The Johns Hopkins Hospital Assistant Professor/Assistant Program Director Columbia University School of Nursing Program in Nurse Anesthesia OVERVIEW • “It’s not the speed which kills, it’s the sudden stop” Epidemiology of Trauma • ~8% worldwide death rate • Leading cause of death in Americans from 1- 45 years of age • MVC’s leading cause of death • Blunt > penetrating • Often drug abusers, acutely intoxicated, HIV and Hepatitis carriers Epidemiology of Trauma • “Golden Hour” – First hour after injury – 50% of patients die within the first seconds to minutesÆ extent of injuries – 30% of patients die in next few hoursÆ major hemorrhage – Rest may die in weeks Æ sepsis, MOSF Pre-hospital Care • ABC’S – Initial assessment and BLS in trauma – GO TEAM: role of CRNA’s at Maryland Shock Trauma Center • Resuscitation • Reduction of fractures • Extrication of trapped victims • Amputation • Uncooperative patients Initial Management Plan • Airway maintenance with cervical spine protection • Breathing: ventilation and oxygenation • Circulation with hemorrhage control • Disability • Exposure Initial Assessment • Primary Survey: – AIRWAY • ALWAYS ASSUME A CERVICAL SPINE INJURY EXISTS UNTIL PROVEN OTHERWISE • Provide MANUAL IN-LINE NECK STABILIZATION • Jaw-thrust maneuver Initial Assessment • Airway cont’d: – Cervical spine evaluation • Cross table lateral and swimmer’s view Xray • Need to see all seven cervical vertebrae • Only negative CT scan R/O injury Initial Assessment • Cervical -

Delayed Traumatic Hemothorax in Older Adults
Open access Brief report Trauma Surg Acute Care Open: first published as 10.1136/tsaco-2020-000626 on 8 March 2021. Downloaded from Complication to consider: delayed traumatic hemothorax in older adults Jeff Choi ,1 Ananya Anand ,1 Katherine D Sborov,2 William Walton,3 Lawrence Chow,4 Oscar Guillamondegui,5 Bradley M Dennis,5 David Spain,1 Kristan Staudenmayer1 ► Additional material is ABSTRACT very small hemothoraces rarely require interven- published online only. To view, Background Emerging evidence suggests older adults tion whereas larger hemothoraces often undergo please visit the journal online immediate drainage. However, emerging evidence (http:// dx. doi. org/ 10. 1136/ may experience subtle hemothoraces that progress tsaco- 2020- 000626). over several days. Delayed progression and delayed suggests HTX in older adults with rib fractures may development of traumatic hemothorax (dHTX) have not experience subtle hemothoraces that progress in a 1Surgery, Stanford University, been well characterized. We hypothesized dHTX would delayed fashion over several days.1 2 If true, older Stanford, California, USA be infrequent but associated with factors that may aid adults may be at risk of developing empyema or 2Vanderbilt University School of Medicine, Nashville, Tennessee, prediction. other complications without close monitoring. USA Methods We retrospectively reviewed adults aged ≥50 Delayed progression and delayed development of 3Radiology, Vanderbilt University years diagnosed with dHTX after rib fractures at two traumatic hemothorax (dHTX) have not been well Medical Center, Nashville, level 1 trauma centers (March 2018 to September 2019). characterized in literature. The ageing US popula- Tennessee, USA tion and increasing incidence of rib fractures among 4Radiology, Stanford University, dHTX was defined as HTX discovered ≥48 hours after Stanford, California, USA admission chest CT showed either no or ’minimal/trace’ older adults underscore a pressing need for better 5Department of Surgery, HTX. -

Management of Traumatic Rib Fractures
GENERAL ANAESTHESIA Tutorial 424 Management of Traumatic Rib Fractures Dr Danny McLaughlin1† 1Anaesthetics Consultant, Royal Cornwall Hospitals NHS Trust, Treliske, Cornwall, UK Edited by: Dr Lara Herbert, Anaesthetics Consultant, Royal Cornwall Hospitals NHS Trust, Treliske, Cornwall, UK † Corresponding author email: [email protected] Published 12 May 2020 KEY POINTS Rib fractures are common sequelae of chest wall trauma. Five or more rib fractures are associated with poorer clinical outcomes. Mortality significantly increases (approximately 30%) when flail chest occurs. Novel fascial plane blocks such as erector spinae blocks are increasingly used for analgesia. INTRODUCTION Rib fractures are common injuries worldwide, often occurring in the context of trauma. These usually occur as a consequence of blunt force trauma to the chest wall, such as that seen in road traffic accidents or falls from a height. However, there are increasing numbers of presentations with injuries following relatively innocuous mechanisms (eg, low-level falls) in older populations. This had led to more focus on so-called ‘silver trauma’ (trauma in older people) to improve trauma care in older patients with increased comorbidities and reduced physiological reserve. Younger patients with isolated rib fractures generally manage with simple analgesia and are less likely to develop serious complications. In contrast, older patients and those with significant comorbidities are at much greater risk of developing respiratory complications such as atelectasis, pneumonia, and subsequent respiratory failure. Individuals with multiple displaced rib fractures and those with a ‘flail’ segment have a significantly increased morbidity and mortality. In these higher risk groups, a coordinated multimodal approach to management with a focus on optimal analgesia and respiratory support is vital to ensuring good outcomes. -

Alabama Trauma Registry (ATR) Web Portal DI Trauma Registry – Tri-Code User Manual
Alabama Trauma Registry (ATR) Web Portal DI Trauma Registry – Tri-Code User Manual Tri-Code Overview ............................................................................................................. 2 Why Code with Tri-Code?.............................................................................................. 2 Using Tri-Code ................................................................................................................... 3 Editing Existing Injury Narrative.................................................................................... 4 Correcting Injury Narrative............................................................................................. 5 Abstracting Injury Descriptions.......................................................................................... 6 Coding Terminology....................................................................................................... 6 ICD9-CM:................................................................................................................... 6 AIS (Abbreviated Injury Scale): ................................................................................. 6 ISS (Injury Severity Score):........................................................................................ 6 RTS (Revised Trauma Score):.................................................................................... 6 Injury Description Entry and Specificity:....................................................................... 6 Spacing:...................................................................................................................... -

Approach to the Trauma Patient Will Help Reduce Errors
The Approach To Trauma Author Credentials Written by: Nicholas E. Kman, MD, The Ohio State University Updated by: Creagh Boulger, MD, and Benjamin M. Ostro, MD, The Ohio State University Last Update: March 2019 Case Study “We have a motor vehicle accident 5 minutes out per EMS report.” 47-year-old male unrestrained driver ejected 15 feet from car arrives via EMS. Vital Signs: BP: 100/40, RR: 28, HR: 110. He was initially combative at the scene but now difficult to arouse. He does not open his eyes, withdrawals only to pain, and makes gurgling sounds. EMS placed a c-collar and backboard, but could not start an IV. What do you do? Objectives Upon completion of this self-study module, you should be able to: ● Describe a focused rapid assessment of the trauma patient using an organized primary and secondary survey. ● Discuss the components of the primary survey. ● Discuss possible pathology that can occur in each domain of the primary survey and recommend treatment/stabilization measures. ● Describe how to stabilize a trauma patient and prioritize resuscitative measures. ● Discuss the secondary survey with particular attention to head/central nervous system (CNS), cervical spine, chest, abdominal, and musculoskeletal trauma. ● Discuss appropriate labs and diagnostic testing in caring for a trauma patient. ● Describe appropriate disposition of a trauma patient. Introduction Nearly 10% of all deaths in the world are caused by injury. Trauma is the number one cause of death in persons 1-50 years of age and results in significant life years lost. According to the National Trauma Data Bank, falls were the leading cause of trauma followed by motor vehicle collisions (MVCs) and firearm related injuries with an overall mortality rate of 4.39% in 2016. -

ICD-10-CM TRAINING November 26, 2013
ICD-10-CM TRAINING November 26, 2013 Injuries, Poisonings, and Certain Consequences of External Causes of Morbidity Linda Dawson, RHIT AHIMA ICD-10-CM/PCS Trainer Seventh Character The biggest change in injury/poisoning coding: The 7th character requirement for each applicable code. Most categories have three 7th character values A – Initial encounter –Patient receiving active treatment for the condition. Surgical treatment, ER, evaluation and treatment by a new physician (Consultant) D- Subsequent encounter - Encounters after the patient has received active treatment. Routine care during the healing or recovery phase. Cast change or removal. Removal of internal or external fixator, medication adjustment, other aftercare follow-up visits following treatment of the injury or condition. S- Sequela - Complications of conditions that arise as a direct result of a conditions, such as scar formations after a burn. The scars are a sequelae of the burn. Initial encounter Patient seen in the Emergency room for initial visit of sprain deltoid ligament R. ankle S93.421A Patient seen by an orthopedic physician in consultation, 2 days after the initial injury for evaluation and care of sprain S93.421A Subsequent encounters : Do not use aftercare codes Injuries or poisonings where 7th characters are provided to identify subsequent care. Subsequent care of injury – Code the acute injury code 7th character “D” for subsequent encounter T23.161D Burn of back of R. hand First degree- visit for dressing change Seventh Character When using the 7th character of “S” use the injury code that precipitated the injury and code for the sequelae. The “S” is added only to the injury code. -

Nuss Procedure for Surgical Stabilization of Flail Chest with Horizontal Sternal Body Fracture and Multiple Bilateral Rib Fractures
Case Report Nuss procedure for surgical stabilization of flail chest with horizontal sternal body fracture and multiple bilateral rib fractures Sung Kwang Lee, Do Kyun Kang Department of Thoracic and Cardiovascular Surgery, Haeundae Paik Hospital, College of Medicine, Inje University, Busan, South Korea Correspondence to: Do Kyun Kang, MD. Department of Thoracic and Cardiovascular Surgery, Haeundae Paik Hospital, College of Medicine, Inje University, 875 (Jwadong) Haeundae-ro, Haeundaegu, Busan 612-030, South Korea. Email: [email protected]. Abstract: Flail chest is a life-threatening situation that paradoxical movement of the thoracic cage was caused by multiply fractured ribs in two different planes, or a sternal fracture, or a combination of the two. The methods to achieve stability of the chest wall are controversy between surgical fixation and mechanical ventilation. We report a case of a 33-year-old man who fell from a high place with fail chest due to multiple rib fractures bilaterally and horizontal sternal fracture. The conventional surgical stabilization using metal plates by access to the front of the sternum could not provide stability of the flail segment because the fracture surface was obliquely upward and there were multiple bilateral rib fractures adjacent the sternum. The Nuss procedure was performed for supporting the flail segment from the back. Flail chest was resolved immediately after the surgery. The patient was weaned from the mechanical ventilation on third postoperative day successfully and was ultimately discharged without any complications. Keywords: Nuss procedure; sternum fracture; rib fractures; flail chest Submitted Feb 13, 2016. Accepted for publication Mar 10, 2016. doi: 10.21037/jtd.2016.04.05 View this article at: http://dx.doi.org/10.21037/jtd.2016.04.05 Introduction the sternal body and bilaterally fractured ribs adjacent to the sternum (Figure 1). -

Chest Injury Advice What Happens When You’Re Admitted Into Hospital with a Chest Injury?
Service: Major Trauma and Emergency Gastrointestinal Surgery Chest Injury Advice What happens when you’re admitted into hospital with a chest injury? Exceptional healthcare, personally delivered Why have you been given this booklet? The reason you have been given this booklet is because you have been diagnosed as having a rib or chest injury. Chest injuries are extremely common following blunt and penetrating trauma. They can vary in severity from minor bruising or an isolated rib fracture to severe crush injuries causing multiple fractures and bleeding which result in pain and breathing problems. Common causes of rib injury include motor vehicle accidents, falls and assaults. Treatment aims to relieve pain allowing you to perform normal tasks while the injury heals. The majority of chest injuries are treated without requiring an operation, but a chest drain may need to be inserted. Occasionally with severe injuries the ribs may have to be fixed. This requires an operation that is performed under general anaesthetic. If you follow the advice given to you in this booklet and by the healthcare professionals on the ward you should find your chest injury much easier to understand and manage. 2 Chest Injury Advice Types of injury (please tick patient’s injury type) Rib fractures A rib fracture is a break in a rib bone. Bruising of the surrounding muscles and ligaments often occurs with these rib fracture. The lungs and other organs underneath the ribs may also be injured. Flail chest A flail chest occurs when a segment of the rib cage is separated from the surrounding structures. -

System Specific Trauma
MODULE 3 System Specific Trauma | Emergency and Essential Surgical Care (EESC) programme 1 www.who.int/surgery OBJECTIVES FOR MODULE 3 To learn specific management strategies for trauma • Head • Spine and spinal cord • Chest • Abdomen • Female genitalia • Musculoskeletal system | Emergency and Essential Surgical Care (EESC) programme 2 www.who.int/surgery Head Injury | Emergency and Essential Surgical Care (EESC) programme 3 www.who.int/surgery HEAD INJURY • Altered level of consciousness is a hallmark of acute cerebral trauma • Never assume that substances (alcohol or drugs) are causes of drowsiness • Frequent clinical mistakes: – Incomplete ABC's, priority management – Incomplete primary, secondary surveys – Incomplete baseline neurologic examination – No reassessment of neurologic status | Emergency and Essential Surgical Care (EESC) programme 4 www.who.int/surgery HEAD INJURY Basal skull fractures – Periorbital ecchymosis (racoon eyes) – Mastoid ecchymosis (Battle's sign) – Cerebrospinal fluid leak from ears or nose Depressed skull fracture – Fragments of skull may penetrate dura, brain Cerebral concussion – Variable temporary altered consciousness | Emergency and Essential Surgical Care (EESC) programme 5 www.who.int/surgery HEAD INJURY Intracerebral hematoma – Caused by acute injury or delayed, progressive bleeding originating from contusion Clinical features of increased intracranial pressure: – Decreased level of consciousness – Bradycardia – Unequal or dilated pupils – Seizures – Focal neurologic deficit | Emergency and Essential -

Trauma to the Thoracic Cage – an Autopsy Study
Medico-legal Update, July-September 2020, Vol.20, No. 3 239 Trauma to the Thoracic Cage – An Autopsy Study Thankamma P. George1, K. Sreekumari2, Sreedevi C. S.3 1Associate Professor and Deputy Police Surgeon, Department of Forensic Medicine, Government Medical College Thiruvananthapuram, 2Joint Director of Medical Education, Directorate of Medical Education, Government of Kerala, Thiruvananthapuram, 3Professor and Police Surgeon, Department of Forensic Medicine, Government Medical College, Trissur Abstract Background: Injuries of the chest causing disruption of the thoracic cage are increasing daily. Thoracic cage may be injured due to blunt trauma like blows, compression of chest or grinding force of automobile run over or due to penetrating injuries. Method: This study analysed the pattern of injury thoracic cage in victims in trauma. 250 cases brought to a tertiary care institution for autopsy were studied from 1st January 2005 to 30th November 2005(1st July 2005-30th November prospectively, and 1st January-30th June 2005, retrospectively). A cross sectional study design including all cases of trauma to the thorax and heart with consecutive sampling was done. Data was collected in pro forma and analysed. Results: 206 victims were males. 130 (52%) were victims of road traffic accidents Pedestrians constituted the majority (26.4%). External injuries were not found in 67 cases (26.8%). Involvement of rib alone constituted 93 cases (37.2 %) followed by combination of rib and lung 54 cases (21.6 %), rib and sternum 13 cases (5.2%), sternum and lung 12 cases (4.8 %) and combination of various other organs occurred in 67 cases (26.8%). Simple fractures were the common rib injury in both sides. -
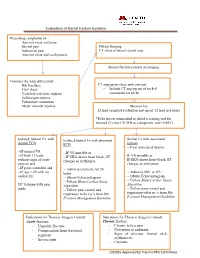
Evaluations of Sternal Fracture Guideline
Evaluations of Sternal Fracture Guideline Presenting complaints of : Anterior chest wall pain Sternal pain Obtain Imaging: Substernal pain CT chest or lateral sternal xray Anterior chest wall ecchymosis Sternal fracture present on imaging Consider the wide differential: Rib fractures, CT angiogram chest with contrast. Flail chest, - Include CT angiogram of neck if Vertebral and spine injuries concurrent 1st rib fx. Solid organ injuries Pulmonary contusions Major vascular injuries. Monitor VS 12 lead on initial evaluation and repeat 12 lead in 6 hours *Echo not recommended as initial screening tool for isolated fx’s nor CK-MB as a diagnostic tool (EAST) Isolated Sternal Fx with Isolated Sternal Fx with abnormal Sternal Fx with associated normal ECG ECG injuries --Treat associated injuries --IF normal VS, --IF VS unstable or --IF both 12 leads --IF EKG shows heart block, ST IF VS unstable or without signs of acute changes or arrthymias IF EKG shows heart block, ST process and changes or arrthymias --IF pain controlled and -- Admit to telemetry for 24 --IF age < 65 with no hours -- Admit to IMC or ICU cardiac hx -- Obtain Echocardiogram -- Obtain Echocardiogram -- Follow Blunt Cardiac Injury -- Follow Blunt Cardiac Injury DC to home with pain Algorithm Algorithm meds -- Follow pain control and -- Follow pain control and respiratory toilet rec’s from Rib respiratory toilet rec’s from Rib Fracture Management Guideline. Fracture Management Guideline Indications for Thoracic Surgery Consult: Indications for Thoracic Surgery Consult: Acute fracture Chronic fracture - Unstable fracture - Chronic severe pain - Compression from fractured - Non-union or malunion. segment - Signs of infection: Sternal click, - Severe pain erythema etc.