Phloroglucinol and Terpenoid Derivatives from Hypericum Cistifolium and H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Antiurolithiatic Plants: Multidimensional Pharmacology
Journal of Pharmacognosy and Phytochemistry 2016; 5(2): 04-24 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2016; 5(2): 04-24 Antiurolithiatic plants: Multidimensional Received: 04-01-2016 Accepted: 06-02-2016 pharmacology Salman Ahmed Lecturer, Department of Salman Ahmed, Muhammad Mohtasheemul Hasan, Zafar Alam Mahmood Pharmacognosy, Faculty of Pharmacy, University of Abstract Karachi, Karachi-75270, Urolithiasis is a common problem afflicted for many centuries with high recurrence. The aim of this Pakistan. review is to provide comprehensive information about traditionally used antiurolithiatic plants and their scientifically proved pharmacological activities like analgesic, anti-inflammatory, antioxidant, astringent, Muhammad Mohtasheemul Hasan demulcent, diuretic, litholytic, lithotriptic, antiurolithiatic, antispasmodic, ACE inhibition and Associate Professor, Department Phospholipase A2 inhibition as a plausible mechanism of action. A total of 503 species, 365 genera and of Pharmacognosy, Faculty of 119 families were cited for treating kidney stones. The most cited families are Asteraceae (41), Fabaceae Pharmacy, University of (34), Lamiaceae (26), Apiaceae (21), Rosaceae (19) and Poaceae (16). The most common used plant Karachi, Karachi-75270, parts are root and rhizome (25%), mode of preparation decoction (62%) and route of administration is Pakistan. oral in all cases. This review will provide the opportunities for the future research and development of new natural antiurolithiatic compounds. Zafar Alam Mahmood Colorcon Limited – UK, Keywords: urolithiasis, antiurolithiatic, natural products, drug development. Flagship House, Victory Way, Crossways, Dartford, Kent, DA26 QD- England. Introduction The belief and observations regarding traditionally used medicinal plants, increasing the interest of people to use natural medicine for their primary health care needs. A wide range of medicinal plants have been used in different countries and cultures as a prophylactic and curative agent for urolithiasis. -

GROUND COVERS for KENTUCKY LANDSCAPES Lenore J
HO-78 C O O P E R A T I V E E X T E N S I O N S E R V I C E U N I V E R S I T Y O F K E N T U C K Y • C O L L E G E O F A G R I C U L T U R E GROUND COVERS FOR KENTUCKY LANDSCAPES Lenore J. Nash, Mary L. Witt,William M. Fountain, Robert L. Geneve “Ground cover” is a term that describes a wide variety Color and texture offered by ground covers give the of plants useful for special planting situations. A common designer additional choices. There is a wide array of characteristic of all ground covers is uniform growth that foliage textures and colors, as well as seasonal flowers and covers the ground with enough density to compete well showy fruit. with weedy plants. Ground covers may function as traffic barriers Naturally-occurring ground covers are a delight, because they do not invite you to walk on them as turf although we may often miss the fact that they are indeed grasses do, yet they are low enough not to be a sight serving as ground covers. Think of mixed assortments of barrier. In this capacity, they give the added benefit of perennial flowers and ferns in a wooded area or snowberry keeping lawnmowers and string trimmers away from blanketing steep slopes along road cuts. valuable woody and herbaceous plants. Ground covers are valuable in special sites where turf grass will not thrive, where regular turf maintenance (mow- Soil ing) is a problem, or where a diversity of color and texture Ground covers grow in close proximity, so well- are desirable. -

The 1700 Native Plants of Bucks County, PA
The 1700 Native Plants of Bucks County, PA Bucks County, PA is blessed with an enormous range of physiographic regions, soil types, and hydrological conditions. Habitats range from the diabase areas of the Upper Bucks to the coastal plains of Lower Bucks, high palisades of the Delaware River to bog remnants, pristine freshwater ponds to tidal areas. These varied conditions host a dizzying array of species, sub‐species, and naturally‐occurring varieties. Common species are regularly available from ArcheWild; many can be grown under contract. Call ArcheWild at 855‐752‐6862 or e‐mail us for more information at: [email protected] Symbol Scientific Name Common Name ACGR2 Acalypha gracilens slender threeseed mercury ACRH Acalypha rhomboidea common threeseed mercury ACVI Acalypha virginica Virginia threeseed mercury ACNE2 Acer negundo boxelder ACNEN Acer negundo var. negundo boxelder ACPE Acer pensylvanicum striped maple ACRU Acer rubrum red maple ACRUR Acer rubrum var. rubrum red maple ACRUT Acer rubrum var. trilobum red maple ACSA2 Acer saccharinum silver maple ACSA3 Acer saccharum sugar maple ACSAS Acer saccharum var. saccharum sugar maple ACSP2 Acer spicatum mountain maple ACMI2 Achillea millefolium common yarrow ACPA Actaea pachypoda white baneberry ACRA7 Actaea racemosa black baneberry ACRAR Actaea racemosa var. racemosa black bugbane ADPE Adiantum pedatum northern maidenhair ADFU Adlumia fungosa allegheny vine AEFL Aesculus flava yellow buckeye AGAU3 Agalinis auriculata earleaf false foxglove AGPU5 Agalinis purpurea purple false foxglove -

St. John's Wort 2018
ONLINE SERIES MONOGRAPHS The Scientific Foundation for Herbal Medicinal Products Hyperici herba St. John's Wort 2018 www.escop.com The Scientific Foundation for Herbal Medicinal Products HYPERICI HERBA St. John's Wort 2018 ESCOP Monographs were first published in loose-leaf form progressively from 1996 to 1999 as Fascicules 1-6, each of 10 monographs © ESCOP 1996, 1997, 1999 Second Edition, completely revised and expanded © ESCOP 2003 Second Edition, Supplement 2009 © ESCOP 2009 ONLINE SERIES ISBN 978-1-901964-61-5 Hyperici herba - St. John's Wort © ESCOP 2018 Published by the European Scientific Cooperative on Phytotherapy (ESCOP) Notaries House, Chapel Street, Exeter EX1 1EZ, United Kingdom www.escop.com All rights reserved Except for the purposes of private study, research, criticism or review no part of this text may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, without the written permission of the publisher. Important Note: Medical knowledge is ever-changing. As new research and clinical experience broaden our knowledge, changes in treatment may be required. In their efforts to provide information on the efficacy and safety of herbal drugs and herbal preparations, presented as a substantial overview together with summaries of relevant data, the authors of the material herein have consulted comprehensive sources believed to be reliable. However, in view of the possibility of human error by the authors or publisher of the work herein, or changes in medical knowledge, neither the authors nor the publisher, nor any other party involved in the preparation of this work, warrants that the information contained herein is in every respect accurate or complete, and they are not responsible for any errors or omissions or for results obtained by the use of such information. -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -

PHYTOCHEMICALS and BIOACTIVITIES of Garcinia Prainiana KING and G
PHYTOCHEMICALS AND BIOACTIVITIES OF Garcinia prainiana KING AND G. hombroniana PIERRE SHAMSUL ON A thesis submitted in fulfilment of the requirements for the award of the degree of Doctor of Philosophy (Chemistry) Faculty of Science Universiti Teknologi Malaysia MARCH 2018 iii To My Beloved Wife Najatulhayah Alwi and My children, Muhd Nabil AnNajat, Muhd Nabihan AnNajat, Muhd Naqib AnNajat, Muhd Nazeem AnNajat, Shahmina Nasyamah AnNajat For Their Love, Support and Best Wishes. iv ACKNOWLEDGEMENT First and foremost, I show my gratitude to The Almighty God for giving me the strength to complete this thesis. I am deeply grateful to everyone who has helped me in completing this work. Thanks a million to my supervisor, Assoc. Prof. Dr. Farediah Ahmad, Prof. Dr. Hasnah Mohd Sirat and Assoc. Prof. Dr. Muhammad Taher for their untiring assistance, direction, encouragement, comments, suggestions, enthusiasm, continuous guidance, ideas, constructive criticism and unrelenting support throughout this work. I would like to thank the Department of Chemistry, Faculty of Science, UTM for the access of UV, IR, GC-MS, and NMR instruments. Sincerely thanks to all lab assistants especially to Mr. Azmi, Mr. Rashidi, Mr. Amin and Mr. Hairol for their help throughout these seven years. Special thanks to my lab mates; Wan Muhd Nuzul, Athirah, Salam, Aminu, Saidu, Shariha, Awanis, Iman, Erni, Edelin, Suri and Yani for their moral support, advice and encouragement to make the lab work meaningful. I am grateful to staff scholarship by Ministry of Higher Education for my doctoral fellowship and Research University Grant (GUP), Universiti Teknologi Malaysia under vote 03H93 for the support throughout the entire research. -

Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Illustrated Flora of East Texas Illustrated Flora of East Texas
ILLUSTRATED FLORA OF EAST TEXAS ILLUSTRATED FLORA OF EAST TEXAS IS PUBLISHED WITH THE SUPPORT OF: MAJOR BENEFACTORS: DAVID GIBSON AND WILL CRENSHAW DISCOVERY FUND U.S. FISH AND WILDLIFE FOUNDATION (NATIONAL PARK SERVICE, USDA FOREST SERVICE) TEXAS PARKS AND WILDLIFE DEPARTMENT SCOTT AND STUART GENTLING BENEFACTORS: NEW DOROTHEA L. LEONHARDT FOUNDATION (ANDREA C. HARKINS) TEMPLE-INLAND FOUNDATION SUMMERLEE FOUNDATION AMON G. CARTER FOUNDATION ROBERT J. O’KENNON PEG & BEN KEITH DORA & GORDON SYLVESTER DAVID & SUE NIVENS NATIVE PLANT SOCIETY OF TEXAS DAVID & MARGARET BAMBERGER GORDON MAY & KAREN WILLIAMSON JACOB & TERESE HERSHEY FOUNDATION INSTITUTIONAL SUPPORT: AUSTIN COLLEGE BOTANICAL RESEARCH INSTITUTE OF TEXAS SID RICHARDSON CAREER DEVELOPMENT FUND OF AUSTIN COLLEGE II OTHER CONTRIBUTORS: ALLDREDGE, LINDA & JACK HOLLEMAN, W.B. PETRUS, ELAINE J. BATTERBAE, SUSAN ROBERTS HOLT, JEAN & DUNCAN PRITCHETT, MARY H. BECK, NELL HUBER, MARY MAUD PRICE, DIANE BECKELMAN, SARA HUDSON, JIM & YONIE PRUESS, WARREN W. BENDER, LYNNE HULTMARK, GORDON & SARAH ROACH, ELIZABETH M. & ALLEN BIBB, NATHAN & BETTIE HUSTON, MELIA ROEBUCK, RICK & VICKI BOSWORTH, TONY JACOBS, BONNIE & LOUIS ROGNLIE, GLORIA & ERIC BOTTONE, LAURA BURKS JAMES, ROI & DEANNA ROUSH, LUCY BROWN, LARRY E. JEFFORDS, RUSSELL M. ROWE, BRIAN BRUSER, III, MR. & MRS. HENRY JOHN, SUE & PHIL ROZELL, JIMMY BURT, HELEN W. JONES, MARY LOU SANDLIN, MIKE CAMPBELL, KATHERINE & CHARLES KAHLE, GAIL SANDLIN, MR. & MRS. WILLIAM CARR, WILLIAM R. KARGES, JOANN SATTERWHITE, BEN CLARY, KAREN KEITH, ELIZABETH & ERIC SCHOENFELD, CARL COCHRAN, JOYCE LANEY, ELEANOR W. SCHULTZE, BETTY DAHLBERG, WALTER G. LAUGHLIN, DR. JAMES E. SCHULZE, PETER & HELEN DALLAS CHAPTER-NPSOT LECHE, BEVERLY SENNHAUSER, KELLY S. DAMEWOOD, LOGAN & ELEANOR LEWIS, PATRICIA SERLING, STEVEN DAMUTH, STEVEN LIGGIO, JOE SHANNON, LEILA HOUSEMAN DAVIS, ELLEN D. -
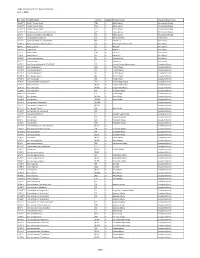
UDBG Inventory of Tree and and Shrubs June 1, 2018 Page 1
UDBG Inventory of Tree and and Shrubs June 1, 2018 Acc. Num Scientific Name Location Mapped Common Name Family Common Name 15-167*3 Abelia 'Canyon Creek' TE2 Y Glossy Abelia Honeysuckle Family 15-167*2 Abelia 'Canyon Creek' TE2 Y Glossy Abelia Honeysuckle Family 15-167*1 Abelia 'Canyon Creek' TE2 Y Glossy Abelia Honeysuckle Family 01-144*1 Abelia x grandiflora [Confetti] = 'Conti' GH Y Glossy Abelia Honeysuckle Family 02-2*1 Abelia x grandiflora 'Little Richard' F5 N Glossy Abelia Honeysuckle Family 70-1*1 Abeliophyllum distichum C3 N White Forsythia Olive Family 15-57*1 Abies balsamea var. phanerolepis NBF Y Balsam Fir Pine Family 90-47*1 Abies cephalonica 'Meyer's Dwarf' C2 N Meyer's Dwarf Grecian Fir Pine Family 89-4*1 Abies concolor C1 N White Fir Pine Family 01-74*1 Abies firma C1 N Momi Fir Pine Family 12-9*7 Abies fraseri PILL N Fraser Fir Pine Family 71-1*2 Abies koreana C1 N Korean Fir Pine Family 95-26*1 Abies nordmanniana C1 N Nordmann Fir Pine Family 96-21*1 Abies pinsapo C1 N Spanish Fir Pine Family 14-72*1 Acer [Crimson Sunset] = 'JFS-KW202' FE Y Crimson Sunset hybrid maple Soapberry Family 88-40*1 Acer buergerianum W2 N Trident Maple Soapberry Family 91-32*1 Acer buergerianum C3 N Trident Maple Soapberry Family 92-78*1 Acer buergerianum A2 Y Trident Maple Soapberry Family 92-78*2 Acer buergerianum A2 Y Trident Maple Soapberry Family 97-43*1 Acer campestre C3 N Hedge Maple Soapberry Family 94-95*1 Acer campsetre 'Compactum' WV1 N Dwarf Hedge Maple Soapberry Family 93-41*1 Acer circinatum NUR5 N Oregon Vine Maple Soapberry Family 93-41*2 Acer circinatum NUR5 N Oregon Vine Maple Soapberry Family 91-58*1 Acer cissifolium F2 Y Ivy-leafed Maple Soapberry Family 98-123*1 Acer davidii FH Y David Maple Soapberry Family 94-13*1 Acer ginnala NUR30 N Amur Maple Soapberry Family 94-13*2 Acer ginnala NUR30 N Amur Maple Soapberry Family 93-56*1 Acer ginnala 'Compactum' NUR29 N Soapberry Family 93-56*2 Acer ginnala 'Compactum' NUR29 N Soapberry Family 97-11*1 Acer ginnala 'Flame' C3 N Amur Maple Soapberry Family 97-13*1 Acer ginnala var. -

Meadow and Woodland Toolkit
Meadow & Woodland Toolkit PEOPLE FOR POLLINATORS EXISTING CONDITIONS People for Pollinators is a 8,700 sq.ft planted mead- particular) but also with regards to aesthetics and the ow surrounded by fencing, with a planted shrub visitor experience. layer on the south side of the fence, adjacent to woodland edges and open fields abutting the Lincoln LLCT’s goals for the site include expanding public Public Schools property. The site is situated on the education and programming; access to the location, northernmost portion of a 10.2-acre site owned and therefore, needs to be more clear and welcoming. protected by LLCT. The soils are mesic and nearly The meadow is currently surrounded by an 8 ft. tall all of the site is in full sun. chain link fence, with only one gate for entry, sit- uated on the northern side. The fence was initially Since 2016, LLCT has managed the site for native installed to prevent deer browse and to deter dog pollinators by direct seeding and planting a variety of forbs, graminoids and shrubs. Approximately 25- 35% of the fenced in meadow remains as non-native grasses and common weeds. After an initial survey of plant species diversity on the site by Evan Abramson and Adam Kohl of Landscape Interactions in 2019, Dr. Gegear sur- veyed the site for bumblebees and at-risk butterflies multiple times in 2020. While pollinator populations at the site were categorized as “high abundance, high diversity” by Dr. Gegear, a lot of room remains for improvement, not only in native plant species diver- sity (early season pollen sources and host plants in 38 LINCOLN POLLINATOR ACTION PLAN Off-Site Emergent Wetland Lincoln Public School Parking Lot PASTURE Path to Site walkers from allowing their dogs off leash. -

Kerry Ann Mendez Jaw-Dropping Flowering Shrubs Sponsored by Bluestone Perennials, Garden Design Magazine and Proven Winners
Kerry Ann Mendez Kennebunk, Maine 207-502-7228 Email: [email protected] Web site: www.pyours.com Jaw-Dropping Flowering Shrubs Sponsored by Bluestone Perennials, Garden Design magazine and Proven Winners *deer resistant 1. General Rules of Thumb for Pruning Flowering Shrubs *Prune spring flowering shrubs right after they bloom *Lilac ‘Red Pixie’ 4’-6’ tall Sun Z 2-7. *Forsythia SHOW OFF ‘Sugar Baby’ Sun – Part Shade 1.5’-2.5’ tall Spring Z 5-8 2. Prune summer and fall flowering shrubs in late winter or early spring while they are still dormant or just breaking dormancy *For variegated plants, remove non-variegated leaf branches *For suckering shrubs (i.e., Forsythia, Kerria, Lilacs), remove some, or all suckers, by cutting the sucker off just beneath the soil surface *Rose of Sharon ‘Sugar Tip’ (Hibiscus syriacus) Sun late Summer 8’-12’ tall Z 5-8 Variegated leaves Sterile 3. Pollarding is when shrubs are cut to the main stem or trunk, ultimately controlling the height of the plants. This is different from coppicing because the trees and shrubs are not cut at ground level, but much higher, usually around six feet. Do this pruning in late winter or early spring while plants are dormant (Horticulture magazine) 4. Coppicing is a pruning technique that shrubs to ground level, causing new shoots to grow rapidly from the base during growing season. Prune hard in late winter or early spring when plants are dormant 5. For each of the featured flowering shrubs I will note pruning recommendations as follows: Prune 1: Prune in late winter or early spring Prune 2: Prune after spring bloom NOTE: Some shrubs seldom need pruning.