Comparative DNA Methylome Analysis of Estrus Ewes Reveals The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification of Key Candidate Genes and Molecular Pathways in White Fat
Pan et al. Human Genomics (2019) 13:55 https://doi.org/10.1186/s40246-019-0239-x PRIMARY RESEARCH Open Access Identification of key candidate genes and molecular pathways in white fat browning: an anti-obesity drug discovery based on computational biology Yuyan Pan, Jiaqi Liu* and Fazhi Qi* Abstract Background: Obesity—with its increased risk of obesity-associated metabolic diseases—has become one of the greatest public health epidemics of the twenty-first century in affluent countries. To date, there are no ideal drugs for treating obesity. Studies have shown that activation of brown adipose tissue (BAT) can promote energy consumption and inhibit obesity, which makes browning of white adipose tissue (WAT) a potential therapeutic target for obesity. Our objective was to identify genes and molecular pathways associated with WAT and the activation of BAT to WAT browning, by using publicly available data and computational tools; this knowledge might help in targeting relevant signaling pathways for treating obesity and other related metabolic diseases. Results: In this study, we used text mining to find out genes related to brown fat and white fat browning. Combined with biological process and pathway analysis in GeneCodis and protein-protein interaction analysis by using STRING and Cytoscape, a list of high priority target genes was developed. The Human Protein Atlas was used to analyze protein expression. Candidate drugs were derived on the basis of the drug-gene interaction analysis of the final genes. Our study identified 18 genes representing 6 different pathways, targetable by a total of 33 drugs as possible drug treatments. The final list included 18 peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists, 4 beta 3 adrenoceptor (β3-AR) agonists, 1 insulin sensitizer, 3 insulins, 6 lipase clearing factor stimulants and other drugs. -

A Clinicopathological and Molecular Genetic Analysis of Low-Grade Glioma in Adults
A CLINICOPATHOLOGICAL AND MOLECULAR GENETIC ANALYSIS OF LOW-GRADE GLIOMA IN ADULTS Presented by ANUSHREE SINGH MSc A thesis submitted in partial fulfilment of the requirements of the University of Wolverhampton for the degree of Doctor of Philosophy Brain Tumour Research Centre Research Institute in Healthcare Sciences Faculty of Science and Engineering University of Wolverhampton November 2014 i DECLARATION This work or any part thereof has not previously been presented in any form to the University or to any other body whether for the purposes of assessment, publication or for any other purpose (unless otherwise indicated). Save for any express acknowledgments, references and/or bibliographies cited in the work, I confirm that the intellectual content of the work is the result of my own efforts and of no other person. The right of Anushree Singh to be identified as author of this work is asserted in accordance with ss.77 and 78 of the Copyright, Designs and Patents Act 1988. At this date copyright is owned by the author. Signature: Anushree Date: 30th November 2014 ii ABSTRACT The aim of the study was to identify molecular markers that can determine progression of low grade glioma. This was done using various approaches such as IDH1 and IDH2 mutation analysis, MGMT methylation analysis, copy number analysis using array comparative genomic hybridisation and identification of differentially expressed miRNAs using miRNA microarray analysis. IDH1 mutation was present at a frequency of 71% in low grade glioma and was identified as an independent marker for improved OS in a multivariate analysis, which confirms the previous findings in low grade glioma studies. -

Comparative Biochemistry and Physiology, Part D, Vol. 5, Pp. 45-54 (2010)
Comparative genomics and proteomics of vertebrate diacylglycerol acyltransferase (DGAT), acyl CoA wax alcohol acyltransferase (AWAT) and monoacylglycerol acyltransferase (MGAT) Author Holmes, Roger S Published 2010 Journal Title Comparative Biochemistry and Physiology, Part D DOI https://doi.org/10.1016/j.cbd.2009.09.004 Copyright Statement © 2010 Elsevier. This is the author-manuscript version of this paper. Reproduced in accordance with the copyright policy of the publisher. Please refer to the journal's website for access to the definitive, published version. Downloaded from http://hdl.handle.net/10072/36786 Griffith Research Online https://research-repository.griffith.edu.au Comparative Biochemistry and Physiology, Part D, Vol. 5, pp. 45-54 (2010) COMPARATIVE GENOMICS AND PROTEOMICS OF VERTEBRATE DIACYLGLYCEROL ACYLTRANSFERASE (DGAT), ACYL CoA WAX ALCOHOL ACYLTRANSFERASE (AWAT) AND MONOACYLGLYCEROL ACYLTRANSFERASE (MGAT) Roger S Holmes School of Biomolecular and Physical Sciences, Griffith University, Nathan 4111 Brisbane Queensland Australia Email: [email protected] Keywords: Diacylglycerol acyltransferase-Monoacylglycerol transferase-Human- Mouse-Opossum-Zebrafish-Genetics-Evolution-X chromosome Running Head: Genomics and proteomics of vertebrate acylglycerol acyltransferases ABSTRACT BLAT (BLAST-Like Alignment Tool) analyses of the opossum (Monodelphis domestica) and zebrafish (Danio rerio) genomes were undertaken using amino acid sequences of the acylglycerol acyltransferase (AGAT) superfamily. Evidence is reported for 8 opossum monoacylglycerol acyltransferase-like (MGAT) (E.C. 2.3.1.22) and diacylglycerol acyltransferase-like (DGAT) (E.C. 2.3.1.20) genes and proteins, including DGAT1, DGAT2, DGAT2L6 (DGAT2-like protein 6), AWAT1 (acyl-CoA wax alcohol acyltransferase 1), AWAT2, MGAT1, MGAT2 and MGAT3. Three of these genes (AWAT1, AWAT2 and DGAT2L6) are closely localized on the opossum X chromosome. -

Proteomics of Lipid Accumulation and DGAT Inhibition in Hepg2 Liver Carcinoma Cells
Proteomics of lipid accumulation and DGAT inhibition in HepG2 liver carcinoma cells. By Bhumika Bhatt-Wessel A thesis submitted to Victoria University of Wellington in fulfilment of the requirement for the degree of Doctor of Philosophy In Cell and Molecular Biology. Victoria University of Wellington 2017. i ABSTRACT Non-alcoholic fatty liver disease (NAFLD) is a manifestation of the metabolic syndrome in the liver. It is marked by hepatocyte accumulation of triacylglycerol (TAG) rich lipid droplets. In some patients, the disease progresses to non-alcoholic steatohepatitis (NASH), characterized by cellular damage, inflammation and fibrosis. In some cases, cirrhosis and liver failure may occur. However, the pathogenesis of NAFLD is still unclear. The present project is based on the hypothesis that hepatocytes are equipped with mechanisms that allow them to manage lipid accumulation to a certain extent. Continued or increased lipid accumulation beyond this triggers molecular mechanisms such as oxidative stress, lipid peroxidation and cell death that aggravate the condition and cause disease progression. The aim of this project is to study the effects of lipid accumulation on the cells using proteomics approach to identify proteins involved in the disease progression. A cell culture model was used in the study. HepG2 cells, a human liver carcinoma cell line, were treated with a mixture of fatty acids (FA) to induce lipid accumulation. The lipid accumulation in HepG2 cells was measured with Oil red O assay and the effect of lipid accumulation on the proliferation of the cells was measured using an MTT cell proliferation assay. HepG2 cells treated with 1 mM FA mixture for 6 hours induced lipid accumulation 1.4 times of control with 90% of cell proliferation capacity of the control cells. -

Supplementary Material Table of Contents
Supplementary M aterial Table of Contents 1 - Online S u pplementary Methods ………………………………………………...… . …2 1.1 Study population……………………………………………………………..2 1.2 Quality control and principal component analysis …………………………..2 1.3 Statistical analyses………………………………………… ………………...3 1.3.1 Disease - specific association testing ……………………………… ..3 1.3.2 Cross - phenotype meta - analysis …………………………………… .3 1.3.3 Model search ……………………………………………………… .4 1.3.4 Novelty of the variants …………………………………………… ..4 1.3.5 Functional Enrichment Analy sis ………………………………… ...4 1.3.6 Drug Target Enrichment Analysis ………………………………… 5 2 - Supplementary Figures………………………………………...………………… . …. 7 3 - Members of the Myositis Genetics Consortium (MYOGEN) ……………………. ..16 4 - Members of the Scleroderma Genetics Consortium ………………… ……………...17 5 - Supplementary References………………………………………………………… . .18 6 - Supplementary Tables………………………………………………………… . ……22 1 Online supplementary m ethods Study population This study was conducted using 12,132 affected subjects and 23 ,260 controls of European des cent population and all of them have been included in previously published GWAS as summarized in Table S1. [1 - 6] Briefly, a total of 3,255 SLE cases and 9,562 ancestry matched controls were included from six countrie s across Europe and North America (Spain, Germany, Netherlands, Italy, UK, and USA). All of the included patients were diagnosed based on the standard American College of Rheumatology (ACR) classification criteria. [7] Previously described GWAS data from 2,363 SSc cases and 5,181 ancestry -

TESI Versió Definitiva Impremta Reedició Corregida 20160330
EVIDÈNCIES EN TEIXIT ADIPÓS D'ALTERACIONS GÈNIQUES DELS VASOS LIMFÀTICS EN L'OBESITAT Alfons Horra Pueyo ADVERTIMENT. L'accés als continguts d'aquesta tesi doctoral i la seva utilització ha de respectar els drets de la persona autora. Pot ser utilitzada per a consulta o estudi personal, així com en activitats o materials d'investigació i docència en els termes establerts a l'art. 32 del Text Refós de la Llei de Propietat Intel·lectual (RDL 1/1996). Per altres utilitzacions es requereix l'autorització prèvia i expressa de la persona autora. En qualsevol cas, en la utilització dels seus continguts caldrà indicar de forma clara el nom i cognoms de la persona autora i el títol de la tesi doctoral. No s'autoritza la seva reproducció o altres formes d'explotació efectuades amb finalitats de lucre ni la seva comunicació pública des d'un lloc aliè al servei TDX. Tampoc s'autoritza la presentació del seu contingut en una finestra o marc aliè a TDX (framing). Aquesta reserva de drets afecta tant als continguts de la tesi com als seus resums i índexs. ADVERTENCIA. El acceso a los contenidos de esta tesis doctoral y su utilización debe respetar los derechos de la persona autora. Puede ser utilizada para consulta o estudio personal, así como en actividades o materiales de investigación y docencia en los términos establecidos en el art. 32 del Texto Refundido de la Ley de Propiedad Intelectual (RDL 1/1996). Para otros usos se requiere la autorización previa y expresa de la persona autora. En cualquier caso, en la utilización de sus contenidos se deberá indicar de forma clara el nombre y apellidos de la persona autora y el título de la tesis doctoral. -

Relative Extended Haplotype Homozygosity Signals Across
et al. Genetics Selection Evolution Bomba (2015) 47:25 Genetics DOI 10.1186/s12711-015-0113-9 Selection Evolution RESEARCH Open Access Relative extended haplotype homozygosity signals across breeds reveal dairy and beef specific signatures of selection Lorenzo Bomba1*†, Ezequiel L Nicolazzi2†, Marco Milanesi1, Riccardo Negrini3, Giordano Mancini4, Filippo Biscarini2, Alessandra Stella2,5, Alessio Valentini6 and Paolo Ajmone-Marsan1 Abstract Background: A number of methods are available to scan a genome for selection signatures by evaluating patterns of diversity within and between breeds. Among these, “extended haplotype homozygosity” (EHH) is a reliable approach to detect genome regions under recent selective pressure. The objective of this study was to use this approach to identify regions that are under recent positive selection and shared by the most representative Italian dairy and beef cattle breeds. Results: A total of 3220 animals from Italian Holstein (2179), Italian Brown (775), Simmental (493), Marchigiana (485) and Piedmontese (379) breeds were genotyped with the Illumina BovineSNP50 BeadChip v.1. After standard quality control procedures, genotypes were phased and core haplotypes were identified. The decay of linkage disequilibrium (LD) for each core haplotype was assessed by measuring the EHH. Since accurate estimates of local recombination rates were not available, relative EHH (rEHH) was calculated for each core haplotype. Genomic regions that carry frequent core haplotypes and with significant rEHH values were considered as candidates for recent positive selection. Candidate regions were aligned across to identify signals shared by dairy or beef cattle breeds. Overall, 82 and 87 common regions were detected among dairy and beef cattle breeds, respectively. -

Genome-Wide Identification of Diacylglycerol Acyltransferases
Liu et al. BMC Genetics (2020) 21:26 https://doi.org/10.1186/s12863-020-0832-y RESEARCH ARTICLE Open Access Genome-wide identification of Diacylglycerol Acyltransferases (DGAT) family genes influencing Milk production in Buffalo Jiajia Liu1,2, Zhiquan Wang3, Jun Li1,4, Hui Li2* and Liguo Yang1* Abstract Background: The diacylglycerol acyltransferases (DGAT) are a vital group of enzymes in catalyzing triacylglycerol biosynthesis. DGAT genes like DGAT1 and DGAT2, have been identified as two functional candidate genes affecting milk production traits, especially for fat content in milk. Buffalo milk is famous for its excellent quality, which is rich in fat and protein content. Therefore, this study aimed to characterize DGAT family genes in buffalo and to find candidate markers or DGAT genes influencing lactation performance. Results: We performed a genome-wide study and identified eight DGAT genes in buffalo. All the DGAT genes classified into two distinct clades (DGAT1 and DGAT2 subfamily) based on their phylogenetic relationships and structural features. Chromosome localization displayed eight buffalo DGAT genes distributed on five chromosomes. Collinearity analysis revealed that the DGAT family genes were extensive homologous between buffalo and cattle. Afterward, we discovered genetic variants loci within the genomic regions that DGAT genes located in buffalo. Seven haplotype blocks were constructed and were associated with buffalo milk production traits. Single marker association analyses revealed four most significant single nucleotide -
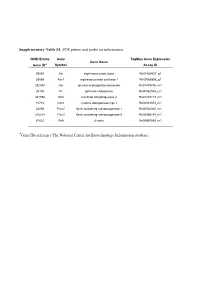
Supplementary Table S1. PCR Primer and Probe Set Information
Supplementary Table S1. PCR primer and probe set information NCBI Entrez Gene TaqMan Gene Expression Gene Name Gene ID* Symbol Assay ID 59085 Asl argininosuccinate lyase Rn01480437_g1 25698 Ass1 argininosuccinate synthase 1 Rn00565808_g1 292804 Gpi glucose-6-phosphate isomerase Rn01475756_m1 25104 Pc pyruvate carboxylase Rn00562534_m1 361596 Idh2 isocitrate dehydrogenase 2 Rn01478119_m1 81718 Cdo1 cysteine dioxygenasetype 1 Rn00583853_m1 25256 Fmo1 flavin containing monooxygenase 1 Rn00562945_m1 246248 Fmo5 flavin containing monooxygenase 5 Rn00595199_m1 81822 Actb β-actin Rn00667869_m1 *Gene IDs reference The National Center for Biotechnology Information database 1 Supplementary Table S2. Metabolite assignments and H NMR data in urine samples of rat* Urine Metabolite δ1H (ppm) 1 N-Methylnicotinamide 4.48(s) 8.18(t) 8.90(d) 8.97(d) 2 Nicotinamide N-oxide 7.73(dd) 8.12(m) 8.48(m) 3 Formate 8.46(s) 4 Hippurate 3.97(d) 7.55(t) 7.64(m) 7.84(dd) 8.56(s) 5 Tryptophan 3.30(dd) 3.47(dd) 4.05(dd) 7.20(m) 7.28(m) 7.50(d) 7.71(d) 6 Phenylalanine 3.11(dd) 3.28(dd) 3.99(dd) 7.36(m) 7.42(m) 7 Tyrosine 3.04(dd) 3.19(dd) 3.93(dd) 6.89(m) 7.17(m) 8 Fumarate 6.53(s) 9 Allantoin 5.39(s) 6.05(s) 7.26(s) 8.01(s) 10 Urea 5.80(s) 11 Glucose 3.24(dd) 3.39(t) 3.40(t) 3.45(m) 3.48(t) 3.53(dd) 3.70(t) 3.72(dd) 3.76(dd) 3.82(m) 3.84(dd) 3.89(dd) 4.65(d) 5.24(d) 12 Tartrate 4.36(s) 13 Threonine 1.33(d) 3.58(d) 4.24(m) 14 Creatinine 3.05(s) 4.06(s) 15 Creatine 3.02(s) 3.95(s) 16 Glycine 3.57(s) 17 Taurine 3.27(t) 3.43(t) 18 Choline 3.20(s) 3.51(m) 4.06(m) 19 cis-Aconitate -

Supplemental Data Inter-Individual Variability in Gene Expression
DMD #42028 Supplemental data Inter-individual variability in gene expression profiles in human hepatocytes and comparison with HepaRG cells Alexandra ROGUE, Carine LAMBERT, Catherine SPIRE, Nancy CLAUDE and André GUILLOUZO Drug and metabolism disposition Supplemental Table 3: Genes expressed only in HepaRG cells located on the chromosome 7 Gene symbol Gene description SEPT13 septin 13 SEPT14 hCG_18833 ACHE acetylcholinesterase (Yt blood group) AMPH amphiphysin ANKIB1 ankyrin repeat and IBR domain containing 1 ANKMY2 ankyrin repeat and MYND domain containing 2 ANLN anillin, actin binding protein AP1S1 adaptor-related protein complex 1, sigma 1 subunit ATXN7L1 ataxin 7-like 1 C1GALT1 core 1 synthase, glycoprotein-N-acetylgalactosamine 3-beta-galactosyltransferase, 1 C7orf31 hCG_39028 C7orf38 chromosome 7 open reading frame 38 C7orf41 chromosome 7 open reading frame 41 C7orf53 chromosome 7 open reading frame 53 CADPS2 Ca++-dependent secretion activator 2 CALCR calcitonin receptor CALN1 calneuron 1 CASD1 CAS1 domain containing 1 CBLL1 Cas-Br-M (murine) ecotropic retroviral transforming sequence-like 1 CCDC132 coiled-coil domain containing 132 CD36 CD36 molecule (thrombospondin receptor) CDC14C CDC14 cell division cycle 14 homolog C (S. cerevisiae) CHN2 chimerin (chimaerin) 2 CHRM2 cholinergic receptor, muscarinic 2 CLDN15 claudin 15 CLIP2 CAP-GLY domain containing linker protein 2 CNPY4 canopy 4 homolog (zebrafish) COX19 COX19 cytochrome c oxidase assembly homolog (S. cerevisiae) CROT carnitine O-octanoyltransferase DBF4 DBF4 homolog (S. cerevisiae) -

Carausius Morosus (Phasmatodea) Homologues of Human Genes with Elevated Expression in the Colon
Ann Colorectal Res. 2019 June; 7(2):e93703. doi: 10.5812/acr.93703. Published online 2019 August 17. Research Article Carausius morosus (Phasmatodea) Homologues of Human Genes with Elevated Expression in the Colon Matan Shelomi 1, * 1Department of Entomology, National Taiwan University, Taipei, Taiwan *Corresponding author: Department of Entomology, National Taiwan University, No. 27, Lane 113, Sec 4, Roosevelt Rd., Taipei, Taiwan. Tel: +886-0233665588, Email: [email protected] Received 2019 May 14; Revised 2019 July 12; Accepted 2019 July 30. Abstract Background: Preliminary testing of novel drugs for colorectal conditions must be performed on animal models, with invertebrate models desirable for practical reasons. The insect excretory organs, the Malpighian tubules, have been cited as models for human renal disease research because they differentially express several genes homologous to those differentially expressed in human kidneys. Their role in excretion and homeostasis suggests that they could be models for human colorectal disease. The insect Ca- rausius morosus (Phasmatodea) has been a model organism for decades. Regarding its potential use as a colorectal disease model, it has an advantage over other insects in that excretion in Phasmatodea is split between two organs: Malpighian tubules and the Phasmatodea-specific “appendices of the midgut”. Objectives: To find homologues of human colon genes expressed in the excretory tissues of C. morosus for potential use in drug testing and other experiments requiring an animal model. Methods: Pre-existing transcriptomics data for the excretory system of the C. morosus were examined to find genes homologous to those known to have elevated expression in the human colon. -

Supplementary Information
SUPPLEMENTARY INFORMATION 1. SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure 1. Long-term exposure to sorafenib increases the expression of progenitor cell-like features. A) mRNA expression levels of PROM-1 (CD133), THY-1 (CD90), EpCAM, KRT19, and VIM assessed by quantitative real-time PCR. Data represent the mean expression value for a gene in each phenotypic type of cells, displayed as fold-changes normalized to 1 (expression value of its corresponding parental non-treated cell line). Expression level is relative to the GAPDH gene. Bars indicate standard deviation. Significant statistical differences are set at p<0.05. B) Immunocitochemical staining of CD90 and vimentin in Hep3B sorafenib resistant cell line and its parental cell line. C) Western blot analysis comparing protein levels in resistant Hu6 and Hep3B cells vs their corresponding parental cells lines. Supplementary Figure 2. Efficacy of gene silencing of IGF1R and FGFR1 and evaluation of MAPK14 signaling activation. IGF1R and FGFR1 knockdown expression 48h after transient transfection with siRNAs (50 nM), in non-treated parental cells and sorafenib-acquired resistant tumor derived cells was assessed by quantitative RT-PCR (A) and western blot (B). C) Activation status of MAPK14 signaling was evaluated by western blot analysis in vivo, in tumors with acquired resistance to sorafenib in comparison to non-treated tumors (right panel), as well as in in vitro, in sorafenib resistant cell lines vs parental non-treated. Supplementary Figure 3. Gene expression levels of several pro-angiogenic factors. mRNA expression levels of FGF1, FGF2, VEGFA, IL8, ANGPT2, KDR, FGFR3, FGFR4 assessed by quantitative real-time PCR in tumors harvested from mice.