Digestive Enzymes of a Small Avian Herbivore, the Rufous-Tailed Plantcutter’
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Threatened Birds of the Americas
PERUVIAN PLANTCUTTER Phytotoma raimondii E1 This rare plantcutter inhabits the coast of northern Peru, where it may require a specific habitat that is now threatened by the almost complete cultivation of the coastal river valleys. DISTRIBUTION The Peruvian Plantcutter is known from very few coastal localities (at altitudes varying from sea-level to 550 m) in Tumbes, Piura, Lambayeque, La Libertad, Ancash and Lima departments, Peru (coordinates, unless otherwise stated, from Stephens and Traylor 1983) as follows: Tumbes Tumbes1 (3°34’S 80°28’W), whence comes the type- specimen, from near sea-level (Taczanowski 1883); Piura Quebrada Salada2 (4°33’S 81°08’W: coordinates given on label), east of Talara, where a specimen (in BMNH) was collected at 90 m in September 1933; near Talara3 (4°33’S 81°13’W), where a bird (in ROM) was taken at 275 m in March 1934; Quebrada Ancha4 (4°36’S 81°08’W: coordinates given on label), east- south-east of Talara, where four birds (in BMNH, ROM) were taken at 170 m in January and October 1933, January 1936 and March 1937; Lambayeque Reque5 (6°52’S 79°50’W), where 20 birds were seen in August 1989 (B. M. Whitney in litt. 1991); Eten6 (6°54’S 79°52’W), whence come six specimens (in BMNH) collected at 10-15 m in September and October 1899; near río Saña7, c.5 km north- north-east of Rafan and c.8 km south-west of Mocupé (the latter being at 7°00’S 79°38’W), where a specimen (in LSUMZ) was collected in September 1978 and where six birds were seen in May 1987 (M. -

Lista Roja De Las Aves Del Uruguay 1
Lista Roja de las Aves del Uruguay 1 Lista Roja de las Aves del Uruguay Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la Unión Internacional para la Conservación de la Naturaleza. Adrián B. Azpiroz, Laboratorio de Genética de la Conservación, Instituto de Investigaciones Biológicas Clemente Estable, Av. Italia 3318 (CP 11600), Montevideo ([email protected]). Matilde Alfaro, Asociación Averaves & Facultad de Ciencias, Universidad de la República, Iguá 4225 (CP 11400), Montevideo ([email protected]). Sebastián Jiménez, Proyecto Albatros y Petreles-Uruguay, Centro de Investigación y Conservación Marina (CICMAR), Avenida Giannattasio Km 30.5. (CP 15008) Canelones, Uruguay; Laboratorio de Recursos Pelágicos, Dirección Nacional de Recursos Acuáticos, Constituyente 1497 (CP 11200), Montevideo ([email protected]). Cita sugerida: Azpiroz, A.B., M. Alfaro y S. Jiménez. 2012. Lista Roja de las Aves del Uruguay. Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la Unión Internacional para la Conservación de la Naturaleza. Dirección Nacional de Medio Ambiente, Montevideo. Descargo de responsabilidad El contenido de esta publicación es responsabilidad de los autores y no refleja necesariamente las opiniones o políticas de la DINAMA ni de las organizaciones auspiciantes y no comprometen a estas instituciones. Las denominaciones empleadas y la forma en que aparecen los datos no implica de parte de DINAMA, ni de las organizaciones auspiciantes o de los autores, juicio alguno sobre la condición jurídica de países, territorios, ciudades, personas, organizaciones, zonas o de sus autoridades, ni sobre la delimitación de sus fronteras o límites. -

Ultimate Bolivia Tour Report 2019
Titicaca Flightless Grebe. Swimming in what exactly? Not the reed-fringed azure lake, that’s for sure (Eustace Barnes) BOLIVIA 8 – 29 SEPTEMBER / 4 OCTOBER 2019 LEADER: EUSTACE BARNES Bolivia, indeed, THE land of parrots as no other, but Cotingas as well and an astonishing variety of those much-loved subfusc and generally elusive denizens of complex uneven surfaces. Over 700 on this tour now! 1 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com Blue-throated Macaws hoping we would clear off and leave them alone (Eustace Barnes) Hopefully, now we hear of colourful endemic macaws, raucous prolific birdlife and innumerable elusive endemic denizens of verdant bromeliad festooned cloud-forests, vast expanses of rainforest, endless marshlands and Chaco woodlands, each ringing to the chorus of a diverse endemic avifauna instead of bleak, freezing landscapes occupied by impoverished unhappy peasants. 2 BirdQuest Tour Report: Ultimate Bolivia 2019 www.birdquest-tours.com That is the flowery prose, but Bolivia IS that great destination. The tour is no longer a series of endless dusty journeys punctuated with miserable truck-stop hotels where you are presented with greasy deep-fried chicken and a sticky pile of glutinous rice every day. The roads are generally good, the hotels are either good or at least characterful (in a good way) and the food rather better than you might find in the UK. The latter perhaps not saying very much. Palkachupe Cotinga in the early morning light brooding young near Apolo (Eustace Barnes). That said, Bolivia has work to do too, as its association with that hapless loser, Che Guevara, corruption, dust and drug smuggling still leaves the country struggling to sell itself. -

Wild Patagonia & Central Chile
WILD PATAGONIA & CENTRAL CHILE: PUMAS, PENGUINS, CONDORS & MORE! October 30 – November 16, 2018 SANTIAGO–HUMBOLDT EXTENSION: ANDES, WETLANDS & ALBATROSS GALORE! November 14-20, 2018 ©2018 Breathtaking Chile! Whether exploring wild Patagonia, watching a Puma hunting a herd of Guanaco against a backdrop of snow-capped spires, enjoying the fascinating antics of a raucous King Penguin colony in Tierra del Fuego, observing a pair of hulking Magellanic Woodpeckers or colorful friendly Tapaculos in a towering Southern Beech forest, or sipping fine wine in a comfortable lodge, this lovely, modern South American country is destined to captivate you! Hosteira Pehoe in Torres Del Paine National Park © Andrew Whittaker Wild Patagonia and Central Chile, Page 2 On this exciting new tour, we will experience the majestic scenery and abundant wildlife of Chile, widely regarded among the most beautiful countries in the world! From Santiago & Talca, in south- central Chile, to the famous Chilean Lake district, charming Chiloe Island to wild Patagonia and Tierra del Fuego in the far south, we will seek out all the special birds, mammals, and vivid landscapes for which the country is justly famous. Our visit is timed for the radiant southern spring when the weather is at its best, colorful blooming wildflowers abound, birds are outfitted in stunning breeding plumage & singing, and photographic opportunities are at their peak. Perhaps most exciting, we will have the opportunity to observe the intimate and poorly known natural history of wild Pumas amid spectacular Torres del Paine National Park, often known as the 8th wonder of the World! Chile is a wonderful place for experiencing nature. -

Ecological Physiology of Diet and Digestive Systems
PH73CH04-Karasov ARI 3 January 2011 9:50 Ecological Physiology of Diet and Digestive Systems William H. Karasov,1,∗ Carlos Martınez´ del Rio,2 and Enrique Caviedes-Vidal3 1Department of Forest and Wildlife Ecology, University of Wisconsin, Madison, Wisconsin 53706; email: [email protected] 2Department of Zoology and Physiology, University of Wyoming, Laramie, Wyoming 82070; email: [email protected] 3Departamento de Bioquımica´ y Ciencias Biologicas,´ Universidad Nacional de San Luis and Instituto Multidisciplinario de Investigaciones Biologicas´ de San Luis, Consejo Nacional de Investigaciones Cientıficas´ y Tecnicas,´ 5700 San Luis, Argentina; email: [email protected] Annu. Rev. Physiol. 2011. 73:69–93 Keywords The Annual Review of Physiology is online at adaptation, hydrolases, transporters, microbiome physiol.annualreviews.org This article’s doi: Abstract 10.1146/annurev-physiol-012110-142152 The morphological and functional design of gastrointestinal tracts of Copyright c 2011 by Annual Reviews. by University of Wyoming on 02/14/11. For personal use only. many vertebrates and invertebrates can be explained largely by the in- All rights reserved teraction between diet chemical constituents and principles of economic 0066-4278/11/0315-0069$20.00 design, both of which are embodied in chemical reactor models of gut Annu. Rev. Physiol. 2011.73:69-93. Downloaded from www.annualreviews.org ∗Corresponding author. function. Natural selection seems to have led to the expression of diges- tive features that approximately match digestive capacities with dietary loads while exhibiting relatively modest excess. Mechanisms explain- ing differences in hydrolase activity between populations and species include gene copy number variations and single-nucleotide polymor- phisms. -
Appendix Ii –Curricula Vitae
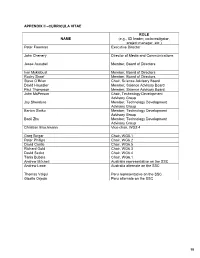
APPENDIX II –CURRICULA VITAE ROL E NAME (e.g., ICI leader, co-investigator, project manager, etc.) Peter Freeman Executive Director John Chenery Director of Media and Communications Jesse Ausubel Member, Board of Directors Ivar Myklebust Member, Board of Directors Rocky Skeef Member, Board of Directors Steve O’Brien Chair, Science Advisory Board David Haussler Member, Science Advisory Board Paul Thompson Member, Science Advisory Board John McPerson Chair, Technology Development Advisory Group Jay Shendure Member, Technology Development Advisory Group Barton Slatko Member, Technology Development Advisory Group Baoli Zhu Member, Technology Development Advisory Group Christian Brochmann Vice-chair, WG2.4 Greg Singer Chair, WG5.1 Peter Phillips Chair, WG6.2 David Castle Chair, WG6.5 Richard Gold Chair, WG6.3 David Secko Chair, WG6.4 Tania Bubela Chair, WG6.1 Andrew Mitchell Australia representative on the SSC Andrew Lowe Australia alternate on the SSC Thomas Valqui Peru representative on the SSC Gisella Orjeda Peru alternate on the SSC 98 PETER FREEMAN BA, PHD, FIBD 120 Stuart Street, Guelph, Ontario, CANADA N1E 4S8 Phone (W): +1 519 824 4120 Cell: +1 519 731 2163 Email: [email protected] PROFILE Experienced science-based (PhD qualified) executive director, with a track record of successfully co- ordinating large multi-institutional research projects, networks and consortia in genomics, proteomics, stem cell research and population health. Strong financial management, strategic planning and project management skills gained in R&D and operations roles in the international malting and brewing industry Superior mentoring, facilitating, technical writing and editing skills used to develop successful interdisciplinary funding proposals. Fully proficient in information /communication technologies and their use in knowledge translation and public outreach activities. -

Federal Register/Vol. 75, No. 2/Tuesday, January 5, 2010
606 Federal Register / Vol. 75, No. 2 / Tuesday, January 5, 2010 / Proposed Rules DEPARTMENT OF THE INTERIOR FOR FURTHER INFORMATION CONTACT: Arlington, VA 22203; telephone 703– Douglas Krofta, Chief, Branch of Listing, 358–2171. Fish and Wildlife Service Endangered Species Program, U.S. Fish Background and Wildlife Service, 4401 N. Fairfax 50 CFR Part 17 Drive, Room 420, Arlington, VA 22203; Section 4(b)(3)(A) of the Act requires telephone 703-358-2105; facsimile 703- us to make a finding (known as a ‘‘90– [Docket No. FWS-R9-IA-2009-0059] day finding’’) on whether a petition to [96100-1671-0000-B6] 358-1735. If you use a telecommunications device for the deaf add a species to, remove a species from, [RIN 1018-AV77] (TDD), call the Federal Information or reclassify a species on the Federal Relay Service (FIRS) at 800-877-8339. Lists of Endangered and Threatened Endangered and Threatened Wildlife Wildlife and Plants has presented SUPPLEMENTARY INFORMATION: and Plants; Listing Foreign Bird substantial information indicating that Species in Peru and Bolivia as Public Comments the requested action may be warranted. Endangered Throughout Their Range We intend that any final action To the maximum extent practicable, we make the finding within 90 days AGENCY: Fish and Wildlife Service, resulting from this proposal will be as Interior. accurate and as effective as possible. following receipt of the petition and publish our finding promptly in the ACTION: Therefore, we request comments or Proposed rule. Federal Register. If we find that the suggestions on this proposed rule. We petition has presented substantial SUMMARY: We, the U.S. -

Developing Tools for Improved Population and Range Estimation in Support of Extinction Risk Assessments for Neotropical Birds
Developing tools for improved population and range estimation in support of extinction risk assessments for Neotropical birds Author Christian Devenish A thesis submitted in partial fulfilment of the requirements of the Manchester Metropolitan University for the degree of Doctor of Philosophy Division of Biology and Conservation Ecology School of Science and the Environment Faculty of Science and Engineering Manchester Metropolitan University January 2017 Cover Photo: Myiarchus semirufus, Gary Rosenberg ii Developing tools for improved population and range estimation in support of extinction risk assessments for Neotropical birds Author Christian Devenish Supervisory team Stuart Marsden, MMU Graeme Buchanan, RSPB Graham Smith, MMU One of the two objects of my Peruvian journey and of our last passage over the Chain of the Andes failed; but on the other hand I had, at the critical moment, the rare good fortune of a perfectly clear day, during a very unfavourable season of the year, on the misty coast of Low Peru. I observed the passage of Mercury over the Sun at Callao, an observation which has become of some importance towards the exact determination of the longitude of Lima, and of all the south-western part of the New Continent. Thus in the intricate relations and graver circumstances of life, there may often be found, associated with disappointment, a germ of compensation. Aspects of Nature. 1849. Alexander von Humboldt iii iv General Abstract Species abundance and distribution metrics are cornerstones of conservation planning, for example, in establishing extinction risk and selecting priority areas, but abundance data are scarce and costly to obtain in comparison to those on species occurrence. -

Cotinga 33 Contents
Cotinga 33 Contents News & Reviews 2 Advertising Information 136 New records of Sulphur-breasted Parakeet Aratinga 3 Club News maculata in Pará and Amapá states, Brazil Thiago Vernaschi Vieira da Costa, Christian Borges Andretti, Fábio Olmos & José 120 Short Communications Fernando Pacheco 120 Nuevos registros de Columbina minuta, Pionus senilis y 137 Marsh Seedeater Sporophila palustris and Tawny-bellied Basileuterus culicivorus en el estado de Yucatán, México Seedeater S. hypoxantha recorded in Tocantins state, Brazil Juan Chablé-Santos, Celia Sélem-Salas & Silvia Hernández- Fábio Olmos & José Fernando Pacheco Betancourt 138 First records of Blue-billed Black Tyrant Knipolegus 121 La Tangara Aliamarilla Thraupis abbas en Costa Rica, cyanirostris for Goiás, Brazil Iubatã Paula de Faria, Sandro historia y dos nuevos registros Andrés Zuñiga & Barata Berg, Tarcísio Lyra dos Santos Abreu, Ana Paula Diniz Luis Sandoval Nakamura & Pedro Diniz 122 Deadly intra-specific aggression in Collared Aracari 140 New data on the breeding biology of Gilt-edged Tanager Pteroglossus torquatus Jeffrey D. Ritterson & Adam C. Stein Tangara cyanoventris Carlos Otávio Araujo Gussoni & Pedro 123 First record of Sungrebe Heliornis fulica on Bonaire, Ferreira Develey Netherlands Antilles Peter J. Rozemeijer 140 Primeiro registro do criticamente ameaçado pica-pau-do- 124 The nest and eggs of Yellow-throated Bush Tanager parnaíba Celeus obrieni no Estado do Mato Grosso (Brasil) Chlorospingus flavigularis Harold F. Greeney, Bryan Suson, e comentários sobre distribuição geográfica e conservação Rudy A. Gelis, Ben Freeman & Eliot T. Miller Túlio Dornas, Gabriel Augusto Leite, Renato Torres Pinheiro & 125 The nest and eggs of Blue-and-black Tanager Tangara Marco Aurélio Crozariol vassorii Harold F. -

Argentina Northern Patagonia, Pampas & Hooded Grebe 1St to 15Th December 2019 (15 Days) North West Patagonia Extension 15Th to 19Th December 2019 (5 Days)
Argentina Northern Patagonia, Pampas & Hooded Grebe 1st to 15th December 2019 (15 days) North West Patagonia Extension 15th to 19th December 2019 (5 days) Hooded Grebe by Luis Segura One of the classic birdwatching destinations, Argentina’s Northern Patagonia and Pampas area not only offers superb birding, but also excellent cuisine, accommodation and transport. A vast country that possesses a large variety of habitats and climates, our tour introduces one to the famous Gaucho ridden Pampas, windswept steppes and endless barren Atlantic shores. Bird diversity thrives here, with almost half of Argentina’s endemics available. The southern Atlantic coasts will provide for some of the most spectacular scenery in the country. We start off with some relaxed city birding around Buenos Aires before heading south, birding the wetlands and tidal mudflats to Punta Rasa. We search for the rare and endangered Yellow Cardinal and range restricted Pampas Meadowlark around Bahía Blanca, while targeting two endemics - White-throated Cacholote and Sandy Gallito around Las Grutas. With a burgeoning list of impressive RBL Argentina - Northern Patagonia & Pampas Itinerary 2 species, we head to what will surely be one of the tour highlights, the magnificent Valdés Peninsula, home of Sea Lion hunting Killer Whales as well as several immense bird and marine mammal breeding grounds. Departing this expansive peninsula, we pay a visit to Punta Tombo where around half a million Magellanic Penguins gather every year to breed in the roughly 200,000 active nests! Finally, we travel to the newly created Patagonia National Park, home to the very attractive, but unfortunately critically endangered, Hooded Grebe. -

Arboreal Perching Birds
Global Federation of Animal Sanctuaries Standards For Arboreal/Perching Bird Sanctuaries Version: December 2019 ©2012 Global Federation of Animal Sanctuaries Global Federation of Animal Sanctuaries – Standards for Arboreal/Perching Bird Sanctuaries Table of Contents INTRODUCTION ............................................................................................................ 1 GFAS PRINCIPLES ............................................................................................................................................................. 1 ANIMALS COVERED BY THESE STANDARDS ................................................................................................. 1 ARBOREAL/PERCHING BIRD STANDARDS .................................................................................................... 3 ARBOREAL/PERCHING BIRD HOUSING ............................................................. 3 H-1. Types of Space and Size .................................................................................................................................................... 3 H-2. Containment ................................................................................................................................................................................ 5 H-3. Ground and Plantings ........................................................................................................................................................... 7 H-4. Gates and Doors ...................................................................................................................................................................... -
The Causes of Avian Extinction and Rarity
The Causes of Avian Extinction and Rarity by Christopher James Lennard Town Cape of University Thesis submitted in the Faculty of Science (Department of Ornithology); University of Cape Town for the degree of Master of Science. June 1997 The University of Cape Town has been given the rllf'lt to reproduce this ~la In whole or In pil't. Copytlght is held by the autl>p'lr . ' The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgementTown of the source. The thesis is to be used for private study or non- commercial research purposes only. Cape Published by the University ofof Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University Declaration: I I certify that this thesis results from my original investigation, except where acknowledged, and has not been submitted for a degree at any other university. Christopher J. Lennard Table of contents Page Table of contents ................................................................................................................ iii List oftigures ................................................................................................................... viii List of tables ....... :............................................................................................................... ix Acknowledgments ..................... , .................................... :.· ................................................. xii Abstract ..............................................................................................................................