The External Validity of Laboratory Experiments: Qualitative Rather Than Quantitative Effects1 by Judd Kessler and Lise Vesterlund
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Internal Validity Is About Causal Interpretability
Internal Validity is about Causal Interpretability Before we can discuss Internal Validity, we have to discuss different types of Internal Validity variables and review causal RH:s and the evidence needed to support them… Every behavior/measure used in a research study is either a ... Constant -- all the participants in the study have the same value on that behavior/measure or a ... • Measured & Manipulated Variables & Constants Variable -- when at least some of the participants in the study • Causes, Effects, Controls & Confounds have different values on that behavior/measure • Components of Internal Validity • “Creating” initial equivalence and every behavior/measure is either … • “Maintaining” ongoing equivalence Measured -- the value of that behavior/measure is obtained by • Interrelationships between Internal Validity & External Validity observation or self-report of the participant (often called “subject constant/variable”) or it is … Manipulated -- the value of that behavior/measure is controlled, delivered, determined, etc., by the researcher (often called “procedural constant/variable”) So, every behavior/measure in any study is one of four types…. constant variable measured measured (subject) measured (subject) constant variable manipulated manipulated manipulated (procedural) constant (procedural) variable Identify each of the following (as one of the four above, duh!)… • Participants reported practicing between 3 and 10 times • All participants were given the same set of words to memorize • Each participant reported they were a Psyc major • Each participant was given either the “homicide” or the “self- defense” vignette to read From before... Circle the manipulated/causal & underline measured/effect variable in each • Causal RH: -- differences in the amount or kind of one behavior cause/produce/create/change/etc. -

Validity and Reliability of the Questionnaire for Compliance with Standard Precaution for Nurses
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Cadernos Espinosanos (E-Journal) Rev Saúde Pública 2015;49:87 Original Articles DOI:10.1590/S0034-8910.2015049005975 Marília Duarte ValimI Validity and reliability of the Maria Helena Palucci MarzialeII Miyeko HayashidaII Questionnaire for Compliance Fernanda Ludmilla Rossi RochaIII with Standard Precaution Jair Lício Ferreira SantosIV ABSTRACT OBJECTIVE: To evaluate the validity and reliability of the Questionnaire for Compliance with Standard Precaution for nurses. METHODS: This methodological study was conducted with 121 nurses from health care facilities in Sao Paulo’s countryside, who were represented by two high-complexity and by three average-complexity health care facilities. Internal consistency was calculated using Cronbach’s alpha and stability was calculated by the intraclass correlation coefficient, through test-retest. Convergent, discriminant, and known-groups construct validity techniques were conducted. RESULTS: The questionnaire was found to be reliable (Cronbach’s alpha: 0.80; intraclass correlation coefficient: (0.97) In regards to the convergent and discriminant construct validity, strong correlation was found between compliance to standard precautions, the perception of a safe environment, and the smaller perception of obstacles to follow such precautions (r = 0.614 and r = 0.537, respectively). The nurses who were trained on the standard precautions and worked on the health care facilities of higher complexity were shown to comply more (p = 0.028 and p = 0.006, respectively). CONCLUSIONS: The Brazilian version of the Questionnaire for I Departamento de Enfermagem. Centro Compliance with Standard Precaution was shown to be valid and reliable. -
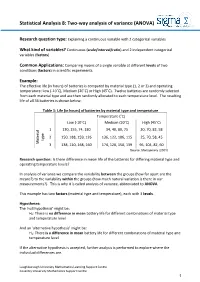
Statistical Analysis 8: Two-Way Analysis of Variance (ANOVA)
Statistical Analysis 8: Two-way analysis of variance (ANOVA) Research question type: Explaining a continuous variable with 2 categorical variables What kind of variables? Continuous (scale/interval/ratio) and 2 independent categorical variables (factors) Common Applications: Comparing means of a single variable at different levels of two conditions (factors) in scientific experiments. Example: The effective life (in hours) of batteries is compared by material type (1, 2 or 3) and operating temperature: Low (-10˚C), Medium (20˚C) or High (45˚C). Twelve batteries are randomly selected from each material type and are then randomly allocated to each temperature level. The resulting life of all 36 batteries is shown below: Table 1: Life (in hours) of batteries by material type and temperature Temperature (˚C) Low (-10˚C) Medium (20˚C) High (45˚C) 1 130, 155, 74, 180 34, 40, 80, 75 20, 70, 82, 58 2 150, 188, 159, 126 136, 122, 106, 115 25, 70, 58, 45 type Material 3 138, 110, 168, 160 174, 120, 150, 139 96, 104, 82, 60 Source: Montgomery (2001) Research question: Is there difference in mean life of the batteries for differing material type and operating temperature levels? In analysis of variance we compare the variability between the groups (how far apart are the means?) to the variability within the groups (how much natural variation is there in our measurements?). This is why it is called analysis of variance, abbreviated to ANOVA. This example has two factors (material type and temperature), each with 3 levels. Hypotheses: The 'null hypothesis' might be: H0: There is no difference in mean battery life for different combinations of material type and temperature level And an 'alternative hypothesis' might be: H1: There is a difference in mean battery life for different combinations of material type and temperature level If the alternative hypothesis is accepted, further analysis is performed to explore where the individual differences are. -

Internal and External Validity: Can You Apply Research Study Results to Your Patients? Cecilia Maria Patino1,2,A, Juliana Carvalho Ferreira1,3,B
J Bras Pneumol. 2018;44(3):183-183 CONTINUING EDUCATION: http://dx.doi.org/10.1590/S1806-37562018000000164 SCIENTIFIC METHODOLOGY Internal and external validity: can you apply research study results to your patients? Cecilia Maria Patino1,2,a, Juliana Carvalho Ferreira1,3,b CLINICAL SCENARIO extent to which the results of a study are generalizable to patients in our daily practice, especially for the population In a multicenter study in France, investigators conducted that the sample is thought to represent. a randomized controlled trial to test the effect of prone vs. supine positioning ventilation on mortality among patients Lack of internal validity implies that the results of the with early, severe ARDS. They showed that prolonged study deviate from the truth, and, therefore, we cannot prone-positioning ventilation decreased 28-day mortality draw any conclusions; hence, if the results of a trial are [hazard ratio (HR) = 0.39; 95% CI: 0.25-0.63].(1) not internally valid, external validity is irrelevant.(2) Lack of external validity implies that the results of the trial may not apply to patients who differ from the study STUDY VALIDITY population and, consequently, could lead to low adoption The validity of a research study refers to how well the of the treatment tested in the trial by other clinicians. results among the study participants represent true fi ndings among similar individuals outside the study. INCREASING VALIDITY OF RESEARCH This concept of validity applies to all types of clinical STUDIES studies, including those about prevalence, associations, interventions, and diagnosis. The validity of a research To increase internal validity, investigators should ensure study includes two domains: internal and external validity. -

Validity and Reliability in Quantitative Studies Evid Based Nurs: First Published As 10.1136/Eb-2015-102129 on 15 May 2015
Research made simple Validity and reliability in quantitative studies Evid Based Nurs: first published as 10.1136/eb-2015-102129 on 15 May 2015. Downloaded from Roberta Heale,1 Alison Twycross2 10.1136/eb-2015-102129 Evidence-based practice includes, in part, implementa- have a high degree of anxiety? In another example, a tion of the findings of well-conducted quality research test of knowledge of medications that requires dosage studies. So being able to critique quantitative research is calculations may instead be testing maths knowledge. 1 School of Nursing, Laurentian an important skill for nurses. Consideration must be There are three types of evidence that can be used to University, Sudbury, Ontario, given not only to the results of the study but also the demonstrate a research instrument has construct Canada 2Faculty of Health and Social rigour of the research. Rigour refers to the extent to validity: which the researchers worked to enhance the quality of Care, London South Bank 1 Homogeneity—meaning that the instrument mea- the studies. In quantitative research, this is achieved University, London, UK sures one construct. through measurement of the validity and reliability.1 2 Convergence—this occurs when the instrument mea- Correspondence to: Validity sures concepts similar to that of other instruments. Dr Roberta Heale, Validity is defined as the extent to which a concept is Although if there are no similar instruments avail- School of Nursing, Laurentian accurately measured in a quantitative study. For able this will not be possible to do. University, Ramsey Lake Road, example, a survey designed to explore depression but Sudbury, Ontario, Canada 3 Theory evidence—this is evident when behaviour is which actually measures anxiety would not be consid- P3E2C6; similar to theoretical propositions of the construct ered valid. -

As an "Odds Ratio", the Ratio of the Odds of Being Exposed by a Case Divided by the Odds of Being Exposed by a Control
STUDY DESIGNS IN BIOMEDICAL RESEARCH VALIDITY & SAMPLE SIZE Validity is an important concept; it involves the assessment against accepted absolute standards, or in a milder form, to see if the evaluation appears to cover its intended target or targets. INFERENCES & VALIDITIES Two major levels of inferences are involved in interpreting a study, a clinical trial The first level concerns Internal validity; the degree to which the investigator draws the correct conclusions about what actually happened in the study. The second level concerns External Validity (also referred to as generalizability or inference); the degree to which these conclusions could be appropriately applied to people and events outside the study. External Validity Internal Validity Truth in Truth in Findings in The Universe The Study The Study Research Question Study Plan Study Data A Simple Example: An experiment on the effect of Vitamin C on the prevention of colds could be simply conducted as follows. A number of n children (the sample size) are randomized; half were each give a 1,000-mg tablet of Vitamin C daily during the test period and form the “experimental group”. The remaining half , who made up the “control group” received “placebo” – an identical tablet containing no Vitamin C – also on a daily basis. At the end, the “Number of colds per child” could be chosen as the outcome/response variable, and the means of the two groups are compared. Assignment of the treatments (factor levels: Vitamin C or Placebo) to the experimental units (children) was performed using a process called “randomization”. The purpose of randomization was to “balance” the characteristics of the children in each of the treatment groups, so that the difference in the response variable, the number of cold episodes per child, can be rightly attributed to the effect of the predictor – the difference between Vitamin C and Placebo. -

Introduction to Difference in Differences (DID) Analysis
Introduction to Difference in Differences (DID) Analysis Hsueh-Sheng Wu CFDR Workshop Series June 15, 2020 1 Outline of Presentation • What is Difference-in-Differences (DID) analysis • Threats to internal and external validity • Compare and contrast three different research designs • Graphic presentation of the DID analysis • Link between regression and DID • Stata -diff- module • Sample Stata codes • Conclusions 2 What Is Difference-in-Differences Analysis • Difference-in-Differences (DID) analysis is a statistic technique that analyzes data from a nonequivalence control group design and makes a casual inference about an independent variable (e.g., an event, treatment, or policy) on an outcome variable • A non-equivalence control group design establishes the temporal order of the independent variable and the dependent variable, so it establishes which variable is the cause and which one is the effect • A non-equivalence control group design does not randomly assign respondents to the treatment or control group, so treatment and control groups may not be equivalent in their characteristics and reactions to the treatment • DID is commonly used to evaluate the outcome of policies or natural events (such as Covid-19) 3 Internal and External Validity • When designing an experiment, researchers need to consider how extraneous variables may threaten the internal validity and external validity of an experiment • Internal validity refers to the extent to which an experiment can establish the causal relation between the independent variable and -

Relations Between Inductive Reasoning and Deductive Reasoning
Journal of Experimental Psychology: © 2010 American Psychological Association Learning, Memory, and Cognition 0278-7393/10/$12.00 DOI: 10.1037/a0018784 2010, Vol. 36, No. 3, 805–812 Relations Between Inductive Reasoning and Deductive Reasoning Evan Heit Caren M. Rotello University of California, Merced University of Massachusetts Amherst One of the most important open questions in reasoning research is how inductive reasoning and deductive reasoning are related. In an effort to address this question, we applied methods and concepts from memory research. We used 2 experiments to examine the effects of logical validity and premise– conclusion similarity on evaluation of arguments. Experiment 1 showed 2 dissociations: For a common set of arguments, deduction judgments were more affected by validity, and induction judgments were more affected by similarity. Moreover, Experiment 2 showed that fast deduction judgments were like induction judgments—in terms of being more influenced by similarity and less influenced by validity, compared with slow deduction judgments. These novel results pose challenges for a 1-process account of reasoning and are interpreted in terms of a 2-process account of reasoning, which was implemented as a multidimensional signal detection model and applied to receiver operating characteristic data. Keywords: reasoning, similarity, mathematical modeling An important open question in reasoning research concerns the arguments (Rips, 2001). This technique can highlight similarities relation between induction and deduction. Typically, individual or differences between induction and deduction that are not con- studies of reasoning have focused on only one task, rather than founded by the use of different materials (Heit, 2007). It also examining how the two are connected (Heit, 2007). -

Questionnaire Validity and Reliability
Questionnaire Validity and reliability Department of Social and Preventive Medicine, Faculty of Medicine Outlines Introduction What is validity and reliability? Types of validity and reliability. How do you measure them? Types of Sampling Methods Sample size calculation G-Power ( Power Analysis) Research • The systematic investigation into and study of materials and sources in order to establish facts and reach new conclusions • In the broadest sense of the word, the research includes gathering of data in order to generate information and establish the facts for the advancement of knowledge. ● Step I: Define the research problem ● Step 2: Developing a research plan & research Design ● Step 3: Define the Variables & Instrument (validity & Reliability) ● Step 4: Sampling & Collecting data ● Step 5: Analysing data ● Step 6: Presenting the findings A questionnaire is • A technique for collecting data in which a respondent provides answers to a series of questions. • The vehicle used to pose the questions that the researcher wants respondents to answer. • The validity of the results depends on the quality of these instruments. • Good questionnaires are difficult to construct; • Bad questionnaires are difficult to analyze. •Identify the goal of your questionnaire •What kind of information do you want to gather with your questionnaire? • What is your main objective? • Is a questionnaire the best way to go about collecting this information? Ch 11 6 How To Obtain Valid Information • Ask purposeful questions • Ask concrete questions • Use time periods -

Reliability, Validity, and Trustworthiness
© Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION Chapter 12 Reliability, Validity, and Trustworthiness James Eldridge © VLADGRIN/iStock/Thinkstock Chapter Objectives At the conclusion of this chapter, the learner will be able to: 1. Identify the need for reliability and validity of instruments used in evidence-based practice. 2. Define reliability and validity. 3. Discuss how reliability and validity affect outcome measures and conclusions of evidence-based research. 4. Develop reliability and validity coefficients for appropriate data. 5. Interpret reliability and validity coefficients of instruments used in evidence-based practice. 6. Describe sensitivity and specificity as related to data analysis. 7. Interpret receiver operand characteristics (ROC) to describe validity. Key Terms Accuracy Content-related validity Concurrent validity Correlation coefficient Consistency Criterion-related validity Construct validity Cross-validation 9781284108958_CH12_Pass03.indd 339 10/20/15 5:59 PM © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION 340 | Chapter 12 Reliability, Validity, and Trustworthiness Equivalency reliability Reliability Interclass reliability Sensitivity Intraclass reliability Specificity Objectivity Stability Observed score Standard error of Predictive validity measurement (SEM) Receiver operand Trustworthiness characteristics (ROC) Validity n Introduction The foundation of good research and of good decision making in evidence- based practice (EBP) is the trustworthiness of the data used to make decisions. When data cannot be trusted, an informed decision cannot be made. Trustworthiness of the data can only be as good as the instruments or tests used to collect the data. Regardless of the specialization of the healthcare provider, nurses make daily decisions on the diagnosis and treatment of a patient based on the results from different tests to which the patient is subjected. -

Randomized Controlled Trials, Development Economics and Policy Making in Developing Countries
Randomized Controlled Trials, Development Economics and Policy Making in Developing Countries Esther Duflo Department of Economics, MIT Co-Director J-PAL [Joint work with Abhijit Banerjee and Michael Kremer] Randomized controlled trials have greatly expanded in the last two decades • Randomized controlled Trials were progressively accepted as a tool for policy evaluation in the US through many battles from the 1970s to the 1990s. • In development, the rapid growth starts after the mid 1990s – Kremer et al, studies on Kenya (1994) – PROGRESA experiment (1997) • Since 2000, the growth have been very rapid. J-PAL | THE ROLE OF RANDOMIZED EVALUATIONS IN INFORMING POLICY 2 Cameron et al (2016): RCT in development Figure 1: Number of Published RCTs 300 250 200 150 100 50 0 1975 1980 1985 1990 1995 2000 2005 2010 2015 Publication Year J-PAL | THE ROLE OF RANDOMIZED EVALUATIONS IN INFORMING POLICY 3 BREAD Affiliates doing RCT Figure 4. Fraction of BREAD Affiliates & Fellows with 1 or more RCTs 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 1980 or earlier 1981-1990 1991-2000 2001-2005 2006-today * Total Number of Fellows and Affiliates is 166. PhD Year J-PAL | THE ROLE OF RANDOMIZED EVALUATIONS IN INFORMING POLICY 4 Top Journals J-PAL | THE ROLE OF RANDOMIZED EVALUATIONS IN INFORMING POLICY 5 Many sectors, many countries J-PAL | THE ROLE OF RANDOMIZED EVALUATIONS IN INFORMING POLICY 6 Why have RCT had so much impact? • Focus on identification of causal effects (across the board) • Assessing External Validity • Observing Unobservables • Data collection • Iterative Experimentation • Unpack impacts J-PAL | THE ROLE OF RANDOMIZED EVALUATIONS IN INFORMING POLICY 7 Focus on Identification… across the board! • The key advantage of RCT was perceived to be a clear identification advantage • With RCT, since those who received a treatment are randomly selected in a relevant sample, any difference between treatment and control must be due to the treatment • Most criticisms of experiment also focus on limits to identification (imperfect randomization, attrition, etc. -

Validity and Reliability in Social Science Research
Education Research and Perspectives, Vol.38, No.1 Validity and Reliability in Social Science Research Ellen A. Drost California State University, Los Angeles Concepts of reliability and validity in social science research are introduced and major methods to assess reliability and validity reviewed with examples from the literature. The thrust of the paper is to provide novice researchers with an understanding of the general problem of validity in social science research and to acquaint them with approaches to developing strong support for the validity of their research. Introduction An important part of social science research is the quantification of human behaviour — that is, using measurement instruments to observe human behaviour. The measurement of human behaviour belongs to the widely accepted positivist view, or empirical- analytic approach, to discern reality (Smallbone & Quinton, 2004). Because most behavioural research takes place within this paradigm, measurement instruments must be valid and reliable. The objective of this paper is to provide insight into these two important concepts, and to introduce the major methods to assess validity and reliability as they relate to behavioural research. The paper has been written for the novice researcher in the social sciences. It presents a broad overview taken from traditional literature, not a critical account of the general problem of validity of research information. The paper is organised as follows. The first section presents what reliability of measurement means and the techniques most frequently used to estimate reliability. Three important questions researchers frequently ask about reliability are discussed: (1) what Address for correspondence: Dr. Ellen Drost, Department of Management, College of Business and Economics, California State University, Los Angeles, 5151 State University Drive, Los Angeles, CA 90032.