Halophytes of the Mediterranean Basin—Underutilized Species with the Potential to Be Nutritious Crops in the Scenario of the Climate Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RESTORATION ACTION PLAN MARINA DUNES PRESERVE Marina, California
RESTORATION ACTION PLAN MARINA DUNES PRESERVE Marina, California Prepared for: Monterey Peninsula Regional Park District 4860 Carmel Valley Road Carmel, CA 93923 Prepared by: Burleson Consulting Inc. 1900 Garden Road, Suite 210 Monterey, CA 93940 March 2021 This page intentionally left blank Restoration Action Plan, Marina Dunes Preserve CONTENTS CONTENTS ..........................................................................................................................................i APPENDICES ...................................................................................................................................... ii ACRONYMS AND ABBREVIATIONS ..................................................................................................... iii 1. INTRODUCTION ...................................................................................................................... 1 1.1 Setting ........................................................................................................................................... 1 1.2 Purpose ......................................................................................................................................... 1 1.3 Approach ....................................................................................................................................... 2 2. UPDATED BEST MANAGEMENT PRACTICES .............................................................................. 3 2.1 Weed Eradication and Control ..................................................................................................... -
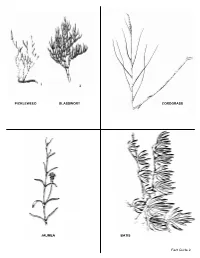
Plant Field Guide
2 PICKLEWEED GLASSWORT CORDGRASS JAUMEA BATIS Field Guide 9 PICKLEWEED Amaranth Family 3 kinds, 2 examples CORDGRASS Grass Family 1 Pickleweed Sarcocornia pacifica Spartina foliosa Glasswort Arthrocnemum subterminalis 2 HABITAT: Growns in the low marsh where the HABITAT: Found throughout the salt marsh. roots are continually bathed in ocean water. APPEARANCE: Stems look like a chain of small APPEARANCE: Look for a tall grass which is pickles. higher than the other plants in the salt marsh. REPRODUCTION: The flowers of all pickleweeds REPRODUCTION: All grasses are wind pollinated. are pollinated by the wind. The small flowers are Look for straw colored spikes of densely packed hard to see because they have no colorful petals flowers. Male flowers will have pollen and the female flowers will show graceful waving stigmas to ADAPTATION TO SALT: Pickleweeds are some of catch the pollen. the many marsh plants that use salt storage (they are accumulators). Also called succulents, these ADAPTATION TO SALT: All the salt marsh plants are swollen with the stored salty water. grasses are salt excreters using special pores to When the salt concentration becomes too high the push out droplets of salty water. Look on the grass cells will die. blades for salt crystals. See sea lavender. ECOLOGICAL RELATIONSHIPS: Frequently the ECOLOGICAL RELATIONSHIPS: Home for the most common plants in the marsh, they provide endangered bird, the Light-footed Clapper Rail. shelter and food for invertebrates. Belding’s A spider lives its whole life inside the blades. Savannah Sparrows build their nests in the Important food for grazing animals. glasswort. BATIS or SALTWORT Saltwort Family Batis maritima HABITAT: Most frequently found in the low marsh. -

Brassicaceae) in Australia
Cunninghamia Date of Publication: 26/08/2013 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) Reassessment of the invasion history of two species of Cakile (Brassicaceae) in Australia Roger D. Cousens, Peter K. Ades, Mohsen B. Mesgaran and Sara Ohadi Melbourne School of Land & Environment, The University of Melbourne, Victoria, 3010, AUSTRALIA Abstract: In this paper we revisit the invasion history of two species of Cakile in Australia. Cakile edentula subsp. edentula arrived in the mid 19th Century and spread into coastal strandline habitat from the southeast towards the west and to the north; Cakile maritima arrived in the late 19th Century and has replaced Cakile edentula over much of the range. While Cakile edentula is morphologically quite uniform, the great variation within Cakile maritima has confused field ecologists. Using herbarium records we update previous accounts of the spread of the species and report on field surveys that determined their current geographic overlap in Tasmania and in northern New South Wales/southern Queensland. We examine regional morphological variation within Cakile maritima using the national herbaria collections and variation within new population samples. We support previous interpretations that Cakile maritima has been introduced on more than one occasion from morphologically distinct races, resulting in regional variation within Australia and high variability within populations in the south-east. Western Australian populations appear distinct and probably did not initiate those in the east; we consider that eastern populations are likely to be a mix of Cakile maritima subsp. maritima from the Mediterranean and Cakile maritima subsp. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

Mesgaran, M. B., Lewis, M. A., Ades, P. K., Donohue, K
Hybridization can facilitate species invasions, even without enhancing local adaptation Mohsen B. Mesgarana, Mark A. Lewisb, Peter K. Adesc, Kathleen Donohued, Sara Ohadia, Chengjun Lia, and Roger D. Cousensa,1 aSchool of BioSciences, The University of Melbourne, Victoria 3010, Australia; bMathematical and Statistical Sciences, University of Alberta, Edmonton, AB, Canada T6G 2G1; cSchool of Ecosystem and Forest Sciences, The University of Melbourne, Victoria 3010, Australia; and dDepartment of Biology, Duke University, Durham, NC 27708 Edited by Alan Hastings, University of California, Davis, CA, and approved July 5, 2016 (received for review April 6, 2016) The founding population in most new species introductions, or at the low pollinator visitation, or both (8, 9); the term “pollen limitation” leading edge of an ongoing invasion, is likely to be small. Severe isoftenusedasagenerictermwhen the exact mechanism is un- Allee effects—reductions in individual fitness at low population den- clear. In the extreme case of a single arriving adult, population sity—may then result in a failure of the species to colonize, even if persistence would normally be impossible unless the species is ca- the habitat could support a much larger population. Using a simula- pable of asexual reproduction or self-fertilization [“Baker’sLaw” tion model for plant populations that incorporates demography, mat- (10)]. Here, we propose a positive role for hybridization in species ing systems, quantitative genetics, and pollinators, we show that invasions and range expansion, a purely demographic mechanism Allee effects can potentially be overcome by transient hybridization without the requirement for any new adaptation to result. with a resident species or an earlier colonizer. -

Characterization of Mycotoxigenic Alternaria Species Isolated from the Tunisian Halophyte Citation: A
Phytopathologia Mediterranea Firenze University Press The international journal of the www.fupress.com/pm Mediterranean Phytopathological Union Research Paper Characterization of mycotoxigenic Alternaria species isolated from the Tunisian halophyte Citation: A. Chalbi, B. Sghaier-Ham- mami, G. Meca, J.M. Quiles, C. Abdel- Cakile maritima ly, C. Marangi, A.F. Logrieco, A. Moret- ti, M. Masiello (2020) Characterization of mycotoxigenic Alternaria species isolated from the Tunisian halophyte Arbia CHALBI1,2,3, Besma SGHAIER-HAMMAMI1,*, Giuseppe MECA4, Cakile maritima. Phytopathologia Medi- Juan Manuel QUILES4, Chedly ABDELLY1, Carmela MARANGI5, Anto- terranea 59(1): 107-118. doi: 10.14601/ nio F. LOGRIECO3, Antonio MORETTI3, Mario MASIELLO3 Phyto-10720 1 Laboratoire des Plantes Extrêmophiles, Centre de Biotechnologie de Borj-Cédria, BP Accepted: February 7, 2020 901, Hammam-Lif 2050, Tunisia 2 Published: April 30, 2020 Faculty of Sciences of Tunis, University Campus 2092, University of Tunis El Manar, Tunis, Tunisia Copyright: © 2020 A. Chalbi, B. 3 Institute of Sciences of Food Production, Research National Council (ISPA-CNR), Via G. Sghaier-Hammami, G. Meca, J.M. Amendola 122/O, 70126, Bari, Italy Quiles, C. Abdelly, C. Marangi, A.F. 4 Laboratory of Food Toxicology, Department of Preventive Medicine, Nutrition and Food Logrieco, A. Moretti, M. Masiello. This Science Area, Faculty of Pharmacy, University of Valencia Avenida Vicent Andres Estelles is an open access, peer-reviewed s/n, 46100 Burjassot, Valencia, Spain article published by Firenze Univer- 5 sity Press (http://www.fupress.com/pm) Institute for Applied Mathematics “M. Picone”, Via G. Amendola 122/D, 70126, Bari, and distributed under the terms of the Italy Creative Commons Attribution License, * Corresponding author: [email protected] which permits unrestricted use, distri- bution, and reproduction in any medi- um, provided the original author and Summary. -

Potential of Halophytes As Source of Edible Oil
ARTICLE IN PRESS Journal of Arid Environments Journal of Arid Environments 68 (2007) 315–321 www.elsevier.com/locate/jnlabr/yjare Short communication Potential of halophytes as source of edible oil D.J. Webera,Ã, R. Ansarib, B. Gulb, M. Ajmal Khanb aDepartment of Integrated Biology, Brigham Young University, Provo, UT 84602, USA bDepartment of Botany, University of Karachi, Karachi 75270, Pakistan Received 15 December 2005; received in revised form 22 May 2006; accepted 23 May 2006 Available online 20 July 2006 Abstract Seeds of Arthrocnemum indicum, Alhaji maurorum, Cressa cretica, Halopyrum mucronatum, Haloxylon stocksii and Suaeda fruticosa were analyzed to determine their potential to be used as source of edible oil. The quantity of oil present varied from 22% to 25%. The amounts of unsaturated fatty acids were high (65–74%) except in A. maurorum. The lipids in the seeds were found to contain 12 unsaturated fatty acids and four saturated fatty acids. The ash content also ranged from 2%–39%. Our data clearly indicate that the seeds of halophytes particularly S. fruticosa could be used as a source of oil for human consumption. r 2006 Elsevier Ltd. All rights reserved. Keywords: Fatty acids; Salinity; Suaeda fruticosa; Seed oil; Saline soils; Ions The demand for vegetable oil in Pakistan has been increasing progressively and has seen rapid growth in this industry from two factory production units in 1947 to more than 40 factory production units in 1998 (Anonymous, 2005). Cottonseed is the major domestic source of edible oil followed by rape, mustard, and canola (Anonymous, 2005). Despite of having a predominantly agrarian economy, Pakistan agriculture is unable to meet the national requirement of vegetable oil. -

Halophytes Energy Feedstocks: Back to Our Roots
The 12th International Symposium on Transport Phenomena and Dynamics of Rotating Machinery Honolulu, Hawaii, February 17–22, 2008 ISROMAC12–2008–20241 HALOPHYTES ENERGY FEEDSTOCKS: BACK TO OUR ROOTS R.C. Hendricks, D.M. Bushnell NASA Glenn Research Center, Cleveland, Ohio 44135, USA 216–977–7507 [email protected] NASA Langley Research Center, Hampton, Virginia 23681, USA 757–864–8987 [email protected] ABSTRACT example, airplanes are not as fuel-flexible as ground Of the Earth’s landmass, ~43% is arid or semi-arid, vehicles, and jet fuel (which is about 6 to 8% of global oil and 97% of the Earth’s water is seawater. Halophytes are consumption) requires high-performance characteristics. salt-tolerant plants (micro and macro) that can prosper in Current biofuel feedstocks and human existence are seawater or brackish waters and are common feedstocks highly dependent on the familiar glycophytes rice, corn, for fuel and food (fuel-food feedstocks) in depressed wheat, potatoes, soy beans, palm oil, and nut plants, which countries. Two types, broadly classed as coastal and desert, cannot tolerate salt. There is some probability that plants can be found in marshes, coastal planes, inland lakes, and started as halophytes, moving from the sea to the shores deserts. Major arid or semi-arid halophyte agriculture and marshes. The not-so-familiar halophytes are highly problems include pumping and draining the required high specialized plants with a great tolerance to salt (see also volumes of irrigation water from sea or ocean sources. app. D). They can germinate, grow, and reproduce in areas Also, not all arid or semi-arid lands are suitable for crops. -

Vascular Plants of the Coastal Dunes of Humboldt County, California
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 4-2019 Vascular Plants of the Coastal Dunes of Humboldt County, California James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Vascular Plants of the Coastal Dunes of Humboldt County, California" (2019). Botanical Studies. 41. https://digitalcommons.humboldt.edu/botany_jps/41 This Flora of Northwest California-Checklists of Local Sites is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. VASCULAR PLANTS OF THE COASTAL DUNES OF HUMBOLDT COUNTY, CALIFORNIA Compiled by James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Arcata, California Ninth Edition • August 2019 Amaryllidaceae — Onion or Amaryllis Family F E R N S Allium unifolium •One-leaved onion Dennstaedtiaceae — Bracken Fern Family Anacardiaceae — Cashew Family Pteridium aquilinum var. pubescens • Bracken fern Toxicodendron diversilobum • Poison-oak Ophioglossaceae — Adder’s-tongue Family Apocynaceae — Dogbane or Milkweed Family Botrychium multifidum • Leathery -

Halophytic Biofuels Revisited
EDITORIAL Halophytic biofuels revisited Biofuels (2013) 4(6), 575–577 At present, more than 40% of Earth is arid or semi-arid, almost 98% of its water is not potable and over “ 800 million ha is already salt affected… ” Bilquees Gul1, Zainul Abideen1, Raziuddin Ansari1 & M Ajmal Khan*2 Keywords: alternate fuel n food security n green energy n salinity n salt tolerant plants Energy availability is central to improvements in econ- production, with both sides having arguments in their omy and agriculture. Fossil fuels, due to their abun- support. Those against it fear reduced supply of food for dance and high density, have been the primary source human consumption and increases in food cost, while of energy; but these resources are finite and may last for others argue that the problem is not of food shortage only the next 50–100 years [1] . Developing technologies but its distribution – there has been excess production at that help to recover oil and gas from deposits previ- certain places and shortage at others even before biofuels ously considered expensive or too difficult to access has were introduced [3]. enabled fuel production to exceed estimates, and has allowed access to new types of reserves, for example, Threat of salinization methane from methane hydrate deposits discovered Resources and supplies of every kind, including those of undersea near Japan and the Arctic East Siberian Sea food, fuel and fiber, are coming under growing pressure [2], but these may only delay the eventuality. In any to meet the demand of 7 billion people and an increas- case, the use of these fossil fuels will result in releasing ing population [4]. -

Intercropping Halophytes to Mitigate Salinity Stress in Watermelon
sustainability Article Intercropping Halophytes to Mitigate Salinity Stress in Watermelon Catherine R. Simpson 1,*, Jose G. Franco 2, Stephen R. King 3 and Astrid Volder 4 1 Texas A&M University-Kingsville, Citrus Center, 312 N International Blvd, Weslaco, TX 78596, USA 2 Northern Great Plains Research Laboratory, United States Department of Agriculture (USDA)-Agricultural Research Service, Mandan, ND 58554, USA; [email protected] 3 Millican Farms LLC, 22168 FM 159, Millican, TX 77866, USA; [email protected] 4 Department of Plant Sciences, University of California-Davis, Davis, CA 95616, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-956-447-3362 Received: 2 February 2018; Accepted: 28 February 2018; Published: 2 March 2018 Abstract: Saline irrigation water can lead to salt buildup and reduced crop yields. Halophytic plants are known to accumulate excess salts in tissues, removing them from the immediate environment. This two-phase experiment explored the feasibility of intercropping watermelon (Citrullus lanatus (Thunb.) Matsum. and Nakai var. lanatus) with halophytic species to mitigate the negative effects of saline irrigation water while providing a value-added crop. In the first experiment, six greenhouse-grown species were irrigated with water that was either deionized (0 dS m−1) or contained 3 or 6 dS m−1 of salts for 41 days and screened for growth and salt removal. Two halophytes were selected to be additively intercropped with watermelon under field conditions and irrigated with the same saline irrigation levels as the first experiment. Results indicated that garden orache (Atriplex hortensis L.) exhibited the highest growth rates and purslane (Portulaca oleracea L.) accumulated high amounts of sodium in plant tissues under saline irrigation.