Agricultural Intensification and Ecosystem Function in a Brigalow (Acacia Harpophylla) Landscape: Implications for Ecosystem Services
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
2021 Land Valuations Overview Western Downs
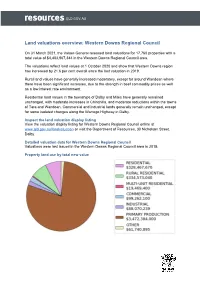
Land valuations overview: Western Downs Regional Council On 31 March 2021, the Valuer-General released land valuations for 17,760 properties with a total value of $4,403,967,344 in the Western Downs Regional Council area. The valuations reflect land values at 1 October 2020 and show that Western Downs region has increased by 21.6 per cent overall since the last valuation in 2019. Rural land values have generally increased moderately, except for around Wandoan where there have been significant increases, due to the strength in beef commodity prices as well as a low interest rate environment. Residential land values in the townships of Dalby and Miles have generally remained unchanged, with moderate increases in Chinchilla, and moderate reductions within the towns of Tara and Wandoan. Commercial and industrial lands generally remain unchanged, except for some isolated changes along the Warrego Highway in Dalby. Inspect the land valuation display listing View the valuation display listing for Western Downs Regional Council online at www.qld.gov.au/landvaluation or visit the Department of Resources, 30 Nicholson Street, Dalby. Detailed valuation data for Western Downs Regional Council Valuations were last issued in the Western Downs Regional Council area in 2019. Property land use by total new value Residential land Table 1 below provides information on median values for residential land within the Western Downs Regional Council area. Table 1 - Median value of residential land Residential Previous New median Change in Number of localities -

Grass Genera in Townsville
Grass Genera in Townsville Nanette B. Hooker Photographs by Chris Gardiner SCHOOL OF MARINE and TROPICAL BIOLOGY JAMES COOK UNIVERSITY TOWNSVILLE QUEENSLAND James Cook University 2012 GRASSES OF THE TOWNSVILLE AREA Welcome to the grasses of the Townsville area. The genera covered in this treatment are those found in the lowland areas around Townsville as far north as Bluewater, south to Alligator Creek and west to the base of Hervey’s Range. Most of these genera will also be found in neighbouring areas although some genera not included may occur in specific habitats. The aim of this book is to provide a description of the grass genera as well as a list of species. The grasses belong to a very widespread and large family called the Poaceae. The original family name Gramineae is used in some publications, in Australia the preferred family name is Poaceae. It is one of the largest flowering plant families of the world, comprising more than 700 genera, and more than 10,000 species. In Australia there are over 1300 species including non-native grasses. In the Townsville area there are more than 220 grass species. The grasses have highly modified flowers arranged in a variety of ways. Because they are highly modified and specialized, there are also many new terms used to describe the various features. Hence there is a lot of terminology that chiefly applies to grasses, but some terms are used also in the sedge family. The basic unit of the grass inflorescence (The flowering part) is the spikelet. The spikelet consists of 1-2 basal glumes (bracts at the base) that subtend 1-many florets or flowers. -

GTA Location Differentials 2020/2021 - QLD Effective 01/10/2020 Further Information - Member Update No
GTA Location Differentials 2020/2021 - QLD Effective 01/10/2020 Further information - Member Update No. 19 of 20 available on the GTA website Location State Port NTP BHC Mode LD ALLORA QLD BRISBANE BRISBANE GRAINX ROAD 19.75 BILOELA QLD GLADSTONE GLADSTONE GRAINCORP ROAD 16.75 BROOKSTEAD QLD BRISBANE BRISBANE GRAINCORP ROAD OR RAIL 22.00 BUNGUNYA QLD BRISBANE BRISBANE GRAINCORP ROAD 41.25 CAPELLA QLD MACKAY MACKAY GRAINCORP ROAD OR RAIL 33.75 CECIL PLAINS QLD BRISBANE BRISBANE QLD COTTON ROAD 27.25 CLIFTON QLD BRISBANE BRISBANE GRAINCORP ROAD 19.75 DALBY WEST QLD BRISBANE BRISBANE GRAINCORP ROAD OR RAIL 24.00 DINGO QLD GLADSTONE GLADSTONE GRAINCORP ROAD 27.00 EMERALD QLD GLADSTONE GLADSTONE GRAINCORP ROAD OR RAIL 36.75 GINDIE QLD GLADSTONE GLADSTONE GRAINCORP ROAD 38.50 GOONDIWINDI QLD BRISBANE BRISBANE CARPENDALE ROAD 35.00 GOONDIWINDI EAST QLD BRISBANE BRISBANE GRAINCORP ROAD OR RAIL 35.00 GOONDIWINDI WEST QLD BRISBANE BRISBANE GRAINCORP ROAD OR RAIL 35.00 JANDOWAE QLD BRISBANE BRISBANE GRAINCORP ROAD 28.00 JONDARYAN QLD BRISBANE BRISBANE CHS BROADBENT ROAD OR RAIL 20.75 KOORNGOO QLD GLADSTONE GLADSTONE GRAINCORP ROAD 18.50 KUPUNN QLD BRISBANE BRISBANE GRAINCORP ROAD 25.75 MACALISTER QLD BRISBANE BRISBANE GRAINCORP ROAD 26.25 MALU QLD BRISBANE BRISBANE GRAINCORP ROAD OR RAIL 21.25 MEANDARRA QLD BRISBANE BRISBANE GRAINCORP ROAD OR RAIL 36.00 MILES QLD BRISBANE BRISBANE GRAINCORP ROAD OR RAIL 34.25 MILLMERRAN QLD BRISBANE BRISBANE GRAINCORP ROAD 23.75 MOURA QLD GLADSTONE GLADSTONE GRAINCORP ROAD 22.00 MT.MCLAREN QLD MACKAY MACKAY GRAINCORP -

Quantitative Ethno-Medicinal Studies of Staple Foods Used by Tribals of Southern Rajasthan (India)
International Journal of Pharmacy and Biological Sciences-IJPBSTM (2019) 9 (1): 908-915 Online ISSN: 2230-7605, Print ISSN: 2321-3272 Research Article | Biological Sciences | Open Access | MCI Approved UGC Approved Journal Quantitative Ethno-Medicinal Studies of Staple Foods Used by Tribals of Southern Rajasthan (India) M Lohar* and A Arora**# *Department of Botany, M L Sukhadia University, Udaipur (Raj). **Department of Botany, B N University, Udaipur (Raj). Received: 10 Oct 2018 / Accepted: 8 Nov 2018 / Published online: 1 Jan 2019 Corresponding Author Email: [email protected] Abstract Ethno-medicinal field study of functional foods with special reference to staple foods carried out in Southern Rajasthan reveals usage of seeds and grains of 16 plants deployed for seven different maladies among which 11 plants are used in diabetes. These staple foods are either consumed as flour / flour additives or boiled as rice. Quantitative analysis for four parameters viz. use value, percent fidelity level, relative index and relative frequency citation reveals maximum dispersion and use of Echinochloa crusgalli by all tribes while Echinochloa colonum and Ipomoea pes-tigridis attributes as a functional millet is least known in studied area. Keywords Southern Rajasthan, Millets, Use value, Percent fidelity level, Relative index, Relative frequency citation, Echinochloa crusgalli ***** INTRODUCTION supplement the diet but should also aid in the In modern voyage a large number of populations is prevention and / or treatment of disease and/or suffering from lifestyle mediated maladies. The disorder”. servings are continuously replaced by short span Among various foods, cereals and millets form an formative junk foods which lack healthy and important food profile as they form the staple food balanced nutritive schedules. -

TAROOM SHOW SOCIETY NEWSLETTER May 2014
TAROOM SHOW SOCIETY NEWSLETTER May 2014 Thank you! The Taroom Show Society would like to sincerely thank everyone who contributed to this year‟s outstanding show- exhibitors, competitors, sponsors, stall holders, families and other visitors. Show president Shane Williams said the 2014 event was a great success, with numerous highlights. “We had the Origin Lumberjack Show, which was an international act and a first for Taroom. The crowd loved it, and the Lumberjacks loved their time in Taroom,” Mr Williams said. “We had the Santos Ladies marquee, the prestigious pet parade, a wine and cheese afternoon, the men‟s chocolate cake competition, plus the traditional Showgirl and Rural Ambassador competitions, just to name a few things.” “We had a huge number of stud cattle compete for what is arguably the largest prize pool in Queensland outside a major city. The Super Bull and Junior Bull Challenges are always a good drawcard. We had over 60 competitors in one show jumping class, making Taroom one of the most popular shows in Queensland. “It was great to see so many people enjoy themselves, and fill the grounds with such a positive vibe. Taroom is such a professionally run show for a small town and it‟s a credit for all those involved,” Mr Williams said. Two volunteers were recognised for their hard work over the years, with life membership being presented to Malcolm and Ann McIntyre. Christie McLennan, 2014 Rural Ambassador Kim Hay, and the 2013 Ian Williams, secretary Tennille Lacey, Miss Show Princess runner-up Queensland Rural Ambassador Jess and president Shane Williams. -

Strategic Plan 2016-2020 Table of Contents
Darling Downs Hospital and Health Service Darling Downs Hospital and Health Service Strategic Plan 2016 2017 update - 2020 Darling Downs Hospital and Health Service Darling Downs Hospital and Health Service Strategic Plan 2016–2020 For further information please contact: Office of the Chief Executive Darling Downs Hospital and Health Service Jofre Level 1 Baillie Henderson Hospital PO Box 405 Toowoomba Qld 4350 [email protected] www.health.qld.gov.au/darlingdowns | ABN 64 109 516 141 Copyright © Darling Downs Hospital and Health Service, The State of Queensland, 2017 This work is licensed under a Creative Commons Attribution Non-Commercial 3.0 Australia licence. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/3.0/au/deed.en/ In essence, you are free to copy, communicate and adapt the work for non-commercial purposes, as long as you attribute Darling Downs Hospital and Health Service and abide by the licence terms. An electronic version of this document is available at www.health.qld.gov.au/about_qhealth/docs/ddhhs-strategic-plan.pdf II Darling Downs Hospital and Health Service | Strategic Plan 2016-2020 Table of contents A message from the Darling Downs Hospital and Health Service Board Chair and Chief Executive ......... 2 Our vision .............................................................................................................................................3 Our values ............................................................................................................................................3 -

Control of Currant Bush (Carissa Ovata) in Developed Brigalow (Acacia Harpophylla) Country
Tropical Grasslands (1998) Volume 32, 259–263 259 Control of currant bush (Carissa ovata) in developed brigalow (Acacia harpophylla) country P.V. BACK can coalesce to cover large areas that signifi- Queensland Beef Industry Institute, Department cantly reduce pasture production. of Primary Industries, Tropical Beef Centre, Ploughing to control brigalow regrowth Rockhampton, Queensland, Australia (Johnson and Back 1974; Scanlan and Anderson 1981) can control currant bush effectively but is very expensive. A more cost-effective treatment is Abstract needed for areas where currant bush dominates in the absence of brigalow regrowth. This paper reports a study designed to test the effectiveness Currant bush (Carissa ovata) is the major native of 6 mechanical methods and 2 herbicide treat- woody weed invading sown buffel grass pastures ments for controlling currant bush in situations in cleared brigalow (Acacia harpophylla) forests where it is the major weed. in Queensland. Stickraking followed by chisel ploughing is a viable alternative to and is more economical than herbicide treatment and blade Materials and methods ploughing for controlling currant bush. Chisel ploughing following stickraking gives good con- Site trol of currant bush with no detrimental effect on existing buffel grass pasture. Stickraking alone is The experiment was carried out on “Tulloch- not sufficient to control currant bush. Ard”, a commercial cattle grazing property 10 km west of Blackwater in central Queensland (23° 33’ S, 148° 44’ E). The original vegetation Introduction comprised a brigalow — blackbutt (Eucalyptus cambageana) scrub with currant bush present in Currant bush (Carissa ovata) is an erect or the understorey, which was cleared and sown to spreading, intricately branched shrub, 1–2 m tall, buffel grass (Cenchrus ciliaris) in 1988. -

Viruses Virus Diseases Poaceae(Gramineae)
Viruses and virus diseases of Poaceae (Gramineae) Viruses The Poaceae are one of the most important plant families in terms of the number of species, worldwide distribution, ecosystems and as ingredients of human and animal food. It is not surprising that they support many parasites including and more than 100 severely pathogenic virus species, of which new ones are being virus diseases regularly described. This book results from the contributions of 150 well-known specialists and presents of for the first time an in-depth look at all the viruses (including the retrotransposons) Poaceae(Gramineae) infesting one plant family. Ta xonomic and agronomic descriptions of the Poaceae are presented, followed by data on molecular and biological characteristics of the viruses and descriptions up to species level. Virus diseases of field grasses (barley, maize, rice, rye, sorghum, sugarcane, triticale and wheats), forage, ornamental, aromatic, wild and lawn Gramineae are largely described and illustrated (32 colour plates). A detailed index Sciences de la vie e) of viruses and taxonomic lists will help readers in their search for information. Foreworded by Marc Van Regenmortel, this book is essential for anyone with an interest in plant pathology especially plant virology, entomology, breeding minea and forecasting. Agronomists will also find this book invaluable. ra The book was coordinated by Hervé Lapierre, previously a researcher at the Institut H. Lapierre, P.-A. Signoret, editors National de la Recherche Agronomique (Versailles-France) and Pierre A. Signoret emeritus eae (G professor and formerly head of the plant pathology department at Ecole Nationale Supérieure ac Agronomique (Montpellier-France). Both have worked from the late 1960’s on virus diseases Po of Poaceae . -

Impacts of Land Clearing
Impacts of Land Clearing on Australian Wildlife in Queensland January 2003 WWF Australia Report Authors: Dr Hal Cogger, Professor Hugh Ford, Dr Christopher Johnson, James Holman & Don Butler. Impacts of Land Clearing on Australian Wildlife in Queensland ABOUT THE AUTHORS Dr Hal Cogger Australasian region” by the Royal Australasian Ornithologists Union. He is a WWF Australia Trustee Dr Hal Cogger is a leading Australian herpetologist and former member of WWF’s Scientific Advisory and author of the definitive Reptiles and Amphibians Panel. of Australia. He is a former Deputy Director of the Australian Museum. He has participated on a range of policy and scientific committees, including the Dr Christopher Johnson Commonwealth Biological Diversity Advisory Committee, Chair of the Australian Biological Dr Chris Johnson is an authority on the ecology and Resources Study, and Chair of the Australasian conservation of Australian marsupials. He has done Reptile & Amphibian Specialist Group (IUCN’s extensive research on herbivorous marsupials of Species Survival Commission). He also held a forests and woodlands, including landmark studies of Conjoint Professorship in the Faculty of Science & the behavioural ecology of kangaroos and wombats, Mathematics at the University of Newcastle (1997- the ecology of rat-kangaroos, and the sociobiology of 2001). He is a member of the International possums. He has also worked on large-scale patterns Commission on Zoological Nomenclature and is a in the distribution and abundance of marsupial past Secretary of the Division of Zoology of the species and the biology of extinction. He is a member International Union of Biological Sciences. He is of the Marsupial and Monotreme Specialist Group of currently the John Evans Memorial Fellow at the the IUCN Species Survival Commission, and has Australian Museum. -

Native Grasses Make New Products – a Review of Current and Past Uses and Assessment of Potential
Native grasses make new products – a review of current and past uses and assessment of potential JUNE 2015 RIRDC Publication No. 15/056 Native grasses make new products A review of current and past uses and assessment of potential by Ian Chivers, Richard Warrick, Janet Bornman and Chris Evans June 2015 RIRDC Publication No 15/056 RIRDC Project No PRJ-009569 © 2015 Rural Industries Research and Development Corporation. All rights reserved. ISBN 978-1-74254-802-9 ISSN 1440-6845 Native grasses make new products: a review of current and past uses and assessment of potential Publication No. 15/056 Project No. PRJ-009569 The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable regions. You must not rely on any information contained in this publication without taking specialist advice relevant to your particular circumstances. While reasonable care has been taken in preparing this publication to ensure that information is true and correct, the Commonwealth of Australia gives no assurance as to the accuracy of any information in this publication. The Commonwealth of Australia, the Rural Industries Research and Development Corporation (RIRDC), the authors or contributors expressly disclaim, to the maximum extent permitted by law, all responsibility and liability to any person, arising directly or indirectly from any act or omission, or for any consequences of any such act or omission, made in reliance on the contents of this publication, whether or not caused by any negligence on the part of the Commonwealth of Australia, RIRDC, the authors or contributors. -

CURRICULUM VITAE Carol Mccormack - Dilga, Glenmorgan, Qld 4423
CURRICULUM VITAE Carol McCormack - Dilga, Glenmorgan, Qld 4423. Tel 07 4665 6798 E: [email protected] W: www.carolmccormack.com.au F: www.facebook.com/carolmccormackartist Art studies 1972 - 2011 Study with various tutors of Flying Arts Inc. including Mervyn Moriarty, Bela Ivanyi, Roy Churcher, Clifton Pugh, Joe Furlonger, Shelagh Morgan, Kim Mahood, Bev Budgen, Wendy Allen, Colin Reaney, Zanette Kahler 2004, 06, 07, 08 Clifton Art masterclasses with Lucja Ray 2008 Quilpie Artist Camp with Noel Miller and Annabel Tully 2010 Professional development workshop with artist Lyne Marshall 2017 January Drawing and painting the figure with Nic Plowman at McGregor Summer School Solo Exhibitions 1986 ‘Landscapes on the Move’ Signatures Gallery, Toowoomba 1987 ‘Bungles, Bunyas and Points Between’, Ardrossan Gallery, Brisbane 1988 ‘Capricorn, Carnarvon & Cobblegun Creek’ Ardrossan Gallery, Brisbane 1989 Shed Gallery, Dalby 1994 ‘Milestones’, Murilla Art Gallery, Miles 1999 Flying Arts Inc solo displays at Law Society Premises, Brisbane, and Australian Institute of Management, Brisbane 2000 ‘Journeys’, a retrospective of 30 years work, curated by Rowena Frost. Balonne River Gallery, Surat 2001 ‘Duo’ – paintings by Carol McCormack and jewellery by Penny Murphy, RM Williams, Toowoomba 2005 ‘More Journeys’ – exhibition of 36 paintings at Balonne River Gallery, Surat 2006 ‘Of this Land’ – exhibition of 30 paintings at Chinchilla White Gums Gallery, Chinchilla 2008 ‘Round & Around’ – exhibition of 32 paintings at Feather & Lawry Design Art Gallery, -

The Roles of the Queensland Herbarium and Its Collections
Proceedings of the 7th and 8th Symposia on Collection Building and Natural History Studies in Asia and the Pacific Rim, edited by Y. Tomida et al., National Science Museum Monographs, (34): 73–81, 2006. The Roles of the Queensland Herbarium and Its Collections Rod J. F. Henderson1, Gordon P. Guymer1 and Paul I. Forster1,2 1Queensland Herbarium, Environmental Protection Agency, Brisbane Botanic Gardens, Mt Coot-tha Road, Toowong, Queensland 4066, Australia (2e-mail: [email protected]) Abstract The roles of the Queensland Herbarium (BRI) and its collections are outlined together with a brief historical account of its founding and subsequent development to the present day. Estab- lished in 1855, the Queensland Herbarium now comprises in excess of 700,000 fully databased botan- ical specimens. The Queensland Herbarium was one of the first herbaria worldwide to database its collections starting in the 1970’s. The Queensland Herbarium (BRI) is the centre for botanical re- search and information on Queensland flora and vegetation communities, and for plant biodiversity research in Queensland’s Environmental Protection Agency (EPA). It is an integral part of the interna- tional network of herbaria, which facilitates research on the Queensland flora by national and interna- tional researchers. The Herbarium is the principal focus for documenting and monitoring Queens- land’s rare and threatened plants and plant communities, vegetation and regional ecosystem surveys, mapping and monitoring, plant names, plant distribution and identification, taxonomic and ecological research on plants and plant communities within the state. Key words: Queensland Herbarium, BRI, Flora. Introduction The Queensland Herbarium (BRI) commenced in 1855, when Walter Hill was appointed Su- perintendent of the Botanic Gardens in Brisbane.