GOA Book.Indb
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Schedule Onlinepdf
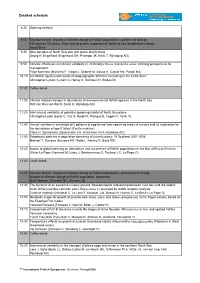
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

Biological Monitoring at Aiktak Island, Alaska in 2016
AMNWR 2017/02 BIOLOGICAL MONITORING AT AIKTAK ISLAND, ALASKA IN 2016 Sarah M. Youngren, Daniel C. Rapp, and Nora A. Rojek Key words: Aiktak Island, Alaska, Aleutian Islands, ancient murrelet, Cepphus columba, common murre, double-crested cormorant, fork-tailed storm-petrel, Fratercula cirrhata, Fratercula corniculata, glaucous-winged gull, horned puffin, Larus glaucescens, Leach’s storm-petrel, Oceanodroma furcata, Oceanodroma leucorhoa, pelagic cormorant, Phalacrocorax auritus, Phalacrocorax pelagicus, Phalacrocorax urile, pigeon guillemot, population trends, productivity, red-faced cormorant, Synthliboramphus antiquus, thick-billed murre, tufted puffin, Uria aalge, Uria lomvia. U.S. Fish and Wildlife Service Alaska Maritime National Wildlife Refuge 95 Sterling Highway, Suite 1 Homer, AK 99603 January 2017 Cite as: Youngren, S. M., D. C. Rapp, and N. A. Rojek. 2017. Biological monitoring at Aiktak Island, Alaska in 2016. U.S. Fish and Wildl. Serv. Rep., AMNWR 2017/02. Homer, Alaska. Tufted puffins flying along the southern coast of Aiktak Island, Alaska. TABLE OF CONTENTS Page INTRODUCTION ........................................................................................................................................... 1 STUDY AREA ............................................................................................................................................... 1 METHODS ................................................................................................................................................... -

Environmental Sensitivity Index Guidelines Version 2.0
NOAA Technical Memorandum NOS ORCA 115 Environmental Sensitivity Index Guidelines Version 2.0 October 1997 Seattle, Washington noaa NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION National Ocean Service Office of Ocean Resources Conservation and Assessment National Ocean Service National Oceanic and Atmospheric Administration U.S. Department of Commerce The Office of Ocean Resources Conservation and Assessment (ORCA) provides decisionmakers comprehensive, scientific information on characteristics of the oceans, coastal areas, and estuaries of the United States of America. The information ranges from strategic, national assessments of coastal and estuarine environmental quality to real-time information for navigation or hazardous materials spill response. Through its National Status and Trends (NS&T) Program, ORCA uses uniform techniques to monitor toxic chemical contamination of bottom-feeding fish, mussels and oysters, and sediments at about 300 locations throughout the United States. A related NS&T Program of directed research examines the relationships between contaminant exposure and indicators of biological responses in fish and shellfish. Through the Hazardous Materials Response and Assessment Division (HAZMAT) Scientific Support Coordination program, ORCA provides critical scientific support for planning and responding to spills of oil or hazardous materials into coastal environments. Technical guidance includes spill trajectory predictions, chemical hazard analyses, and assessments of the sensitivity of marine and estuarine environments to spills. To fulfill the responsibilities of the Secretary of Commerce as a trustee for living marine resources, HAZMAT’s Coastal Resource Coordination program provides technical support to the U.S. Environmental Protection Agency during all phases of the remedial process to protect the environment and restore natural resources at hundreds of waste sites each year. -

Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries
JANUARY 2020 Wholesale Market Profiles for Alaska Groundfish and FisheriesCrab Wholesale Market Profiles for Alaska Groundfish and Crab Fisheries JANUARY 2020 JANUARY Prepared by: McDowell Group Authors and Contributions: From NOAA-NMFS’ Alaska Fisheries Science Center: Ben Fissel (PI, project oversight, project design, and editor), Brian Garber-Yonts (editor). From McDowell Group, Inc.: Jim Calvin (project oversight and editor), Dan Lesh (lead author/ analyst), Garrett Evridge (author/analyst) , Joe Jacobson (author/analyst), Paul Strickler (author/analyst). From Pacific States Marine Fisheries Commission: Bob Ryznar (project oversight and sub-contractor management), Jean Lee (data compilation and analysis) This report was produced and funded by the NOAA-NMFS’ Alaska Fisheries Science Center. Funding was awarded through a competitive contract to the Pacific States Marine Fisheries Commission and McDowell Group, Inc. The analysis was conducted during the winter of 2018 and spring of 2019, based primarily on 2017 harvest and market data. A final review by staff from NOAA-NMFS’ Alaska Fisheries Science Center was completed in June 2019 and the document was finalized in March 2016. Data throughout the report was compiled in November 2018. Revisions to source data after this time may not be reflect in this report. Typically, revisions to economic fisheries data are not substantial and data presented here accurately reflects the trends in the analyzed markets. For data sourced from NMFS and AKFIN the reader should refer to the Economic Status Report of the Groundfish Fisheries Off Alaska, 2017 (https://www.fisheries.noaa.gov/resource/data/2017-economic-status-groundfish-fisheries-alaska) and Economic Status Report of the BSAI King and Tanner Crab Fisheries Off Alaska, 2018 (https://www.fisheries.noaa. -

Research Article Early Development of Monoplex Pilearis
1 Research Article 2 Early Development of Monoplex pilearis and Monoplex parthenopeus (Gastropoda: 3 Cymatiidae) - Biology and Morphology 4 5 Ashlin H. Turner*, Quentin Kaas, David J. Craik, and Christina I. Schroeder* 6 7 Institute for Molecular Bioscience, The University of Queensland, Brisbane, 4072, Qld, Australia 8 9 *Corresponding authors: 10 Email: [email protected], phone: +61-7-3346-2023 11 Email: [email protected], phone: +61-7-3346-2021 1 12 Abstract 13 Members of family Cymatiidae have an unusually long planktonic larval life stage (veligers) which 14 allows them to be carried within ocean currents and become distributed worldwide. However, little 15 is known about these planktonic veligers and identification of the larval state of many Cymatiidae 16 is challenging at best. Here we describe the first high-quality scanning electron microscopy images 17 of the developing veliger larvae of Monoplex pilearis and Monoplex parthenopeus (Gastropoda: 18 Cymatiidae). The developing shell of Monoplex veligers was captured by SEM, showing plates 19 secreted to form the completed shell. The incubation time of the two species was recorded and 20 found to be different; M. parthenopeus took 24 days to develop fully and hatch out of the egg 21 capsules, whereas M. pilearis took over a month to leave the egg capsule. Using scanning electron 22 microscopy and geometric morphometrics, the morphology of veliger larvae was compared. No 23 significant differences were found between the shapes of the developing shell between the two 24 species; however, it was found that M. pilearis was significantly larger than M. -

Introduction
AMNWR 08/13 BIOLOGICAL MONITORING AT AIKTAK ISLAND, ALASKA IN 2008: SUMMARY APPENDICES Brie A. Drummond Key words: Aiktak Island, Alaska, Aleutian Islands, ancient murrelet, Cepphus columba, common murre, double-crested cormorant, fork-tailed storm-petrel, Fratercula cirrhata, Fratercula corniculata, glaucous-winged gull, horned puffin, Larus glaucescens, Leach’s storm-petrel, Oceanodroma leucorhoa, Oceanodroma furcata, pelagic cormorant, Phalacrocorax auritus, Phalacrocorax pelagicus, Phalacrocorax urile, pigeon guillemot, population trends, productivity, red-faced cormorant, Synthliboramphus antiquus, thick-billed murre, tufted puffin, Uria aalge, Uria lomvia U.S. Fish and Wildlife Service Alaska Maritime National Wildlife Refuge Aleutian Islands Unit 95 Sterling Hwy, Suite 1 Homer, Alaska 99603 September 2008 Cite as: Drummond, B. A. Biological monitoring at Aiktak Island, Alaska in 2008: summary appendices. U.S. Fish and Wildl. Serv. Rep., AMNWR 08/13. Homer, Alaska. 139 pp. Southern coast of Aiktak Island, Alaska, in early June. TABLE OF CONTENTS PAGE INTRODUCTION.......................................................................................................................................... 1 STUDY AREA............................................................................................................................................. ..1 METHODS.................................................................................................................................................. ..2 INTERESTING OBSERVATIONS.............................................................................................................. -

Aleutian Islands
Journal of Global Change Data & Discovery. 2018, 2(1): 109-114 © 2018 GCdataPR DOI:10.3974/geodp.2018.01.18 Global Change Research Data Publishing & Repository www.geodoi.ac.cn Global Change Data Encyclopedia Aleutian Islands Liu, C.1* Yang, A. Q.2 Hu, W. Y.1 Liu, R. G.1 Shi, R. X.1 1. Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing 100101, China; 2. Institute of Remote Sensing and Digital Earth,Chinese Academy of Sciences,Beijing100101,China Keywords: Aleutian Islands; Fox Islands; Four Mountains Islands; Andreanof Islands; Rat Islands; Near Islands; Kommandor Islands; Unimak Island; USA; Russia; data encyclopedia The Aleutian Islands extends latitude from 51°12′35″N to 55°22′14″N and longitude about 32 degrees from 165°45′10″E to 162°21′10″W, it is a chain volcanic islands belonging to both the United States and Russia[1–3] (Figure 1, 2). The islands are formed in the northern part of the Pacific Ring of Fire. They form part of the Aleutian Arc in the Northern Pacific Ocean, extending about 1,900 km westward from the Alaska Peninsula to- ward the Kamchatka Peninsula in Russia, Figure 1 Dataset of Aleutian Islands in .kmz format and mark a dividing line between the Ber- ing Sea to the north and the Pacific Ocean to the south. The islands comprise 6 groups of islands (east to west): the Fox Islands[4–5], islands of Four Mountains[6–7], Andreanof Islands[8–9], Rat Islands[10–11], Near Is- lands[12–13] and Kommandor Islands[14–15]. -

Foregut Anatomy of the Cochlespirinae (Gastropoda, Conoidea, Turridae)
Foregut anatomy of the Cochlespirinae (Gastropoda, Conoidea, Turridae) Alexandra I. MEDINSKAYA A. N. Severtzov Institute of Problems of Evolution, Leninsky Prospect 33, Moscow 117071 (Russia) Medinskaya A. I. 1999. — Foregut anatomy of the Cochlespirinae (Gastropoda. Conoidea. Turridae). Zoosystema2\ (2): 171-198. ABSTRACT The foregut anatomy of 20 species, belonging to eight genera, of the sub family Cochlespirinae is described. A cladistic analysis based on several most important characters (morphology of proboscis, position of buccal sphinc ters, histology of venom gland, position of the venom gland opening, struc ture of muscular bulb, and morphology of radular teeth) revealed three more or less well-defined groups within the subfamily. The main feature characte rizing the subfamily as a whole and separating groups within it, appeared to be the structure of venom gland and its muscular bulb. The subgenus KEYWORDS Cochlespirinae, Sibogasyrinx of the genus Leucosyrinx was shown to deserve a genus status. Conoidea, Some genera appeared to be intermediate between Cochlespirinae and anatomy, foregut, Crassispirinae in some anatomical characters, and their taxonomic position histology. remains not completely clear. RESUME L'anatomie du système digestif des Cochlespirinae (Gastropoda, Conoidea, Turridae). L'anatomie du système digestif de 20 espèces, appartenant à huit genres de la sous-famille Cochlespirinae, est étudiée. Une analyse cladistique, fondée sur les plus importants caractères de ce groupe (la morphologie de la trompe, la disposition des sphincters, l'histologie de la glande à venin, la disposition de l'ouverture de la glande à venin, la structure de la poire musculaire et la mor phologie des dents de la radula) a permis de distinguet trois groupes plus ou moins homogènes. -

XIV. Appendices
Appendix 1, Page 1 XIV. Appendices Appendix 1. Vertebrate Species of Alaska1 * Threatened/Endangered Fishes Scientific Name Common Name Eptatretus deani black hagfish Lampetra tridentata Pacific lamprey Lampetra camtschatica Arctic lamprey Lampetra alaskense Alaskan brook lamprey Lampetra ayresii river lamprey Lampetra richardsoni western brook lamprey Hydrolagus colliei spotted ratfish Prionace glauca blue shark Apristurus brunneus brown cat shark Lamna ditropis salmon shark Carcharodon carcharias white shark Cetorhinus maximus basking shark Hexanchus griseus bluntnose sixgill shark Somniosus pacificus Pacific sleeper shark Squalus acanthias spiny dogfish Raja binoculata big skate Raja rhina longnose skate Bathyraja parmifera Alaska skate Bathyraja aleutica Aleutian skate Bathyraja interrupta sandpaper skate Bathyraja lindbergi Commander skate Bathyraja abyssicola deepsea skate Bathyraja maculata whiteblotched skate Bathyraja minispinosa whitebrow skate Bathyraja trachura roughtail skate Bathyraja taranetzi mud skate Bathyraja violacea Okhotsk skate Acipenser medirostris green sturgeon Acipenser transmontanus white sturgeon Polyacanthonotus challengeri longnose tapirfish Synaphobranchus affinis slope cutthroat eel Histiobranchus bathybius deepwater cutthroat eel Avocettina infans blackline snipe eel Nemichthys scolopaceus slender snipe eel Alosa sapidissima American shad Clupea pallasii Pacific herring 1 This appendix lists the vertebrate species of Alaska, but it does not include subspecies, even though some of those are featured in the CWCS. -

Miocene Vetigastropoda and Neritimorpha (Mollusca, Gastropoda) of Central Chile
Journal of South American Earth Sciences 17 (2004) 73–88 www.elsevier.com/locate/jsames Miocene Vetigastropoda and Neritimorpha (Mollusca, Gastropoda) of central Chile Sven N. Nielsena,*, Daniel Frassinettib, Klaus Bandela aGeologisch-Pala¨ontologisches Institut und Museum, Universita¨t Hamburg, Bundesstrasse 55, 20146 Hamburg, Germany bMuseo Nacional de Historia Natural, Casilla 787, Santiago, Chile Abstract Species of Vetigastropoda (Fissurellidae, Turbinidae, Trochidae) and one species of Neritimorpha (Neritidae) from the Navidad area, south of Valparaı´so, and the Arauco Peninsula, south of Concepcio´n, are described. Among these, the Fissurellidae comprise Diodora fragilis n. sp., Diodora pupuyana n. sp., two additional unnamed species of Diodora, and a species resembling Fissurellidea. Turbinidae are represented by Cantrainea sp., and Trochidae include Tegula (Chlorostoma) austropacifica n. sp., Tegula (Chlorostoma) chilena n. sp., Tegula (Chlorostoma) matanzensis n. sp., Tegula (Agathistoma) antiqua n. sp., Bathybembix mcleani n. sp., Gibbula poeppigii [Philippi, 1887] n. comb., Diloma miocenica n. sp., Fagnastesia venefica [Philippi, 1887] n. gen. n. comb., Fagnastesia matanzana n. gen. n. sp., Calliostoma mapucherum n. sp., Calliostoma kleppi n. sp., Calliostoma covacevichi n. sp., Astele laevis [Sowerby, 1846] n. comb., and Monilea riorapelensis n. sp. The Neritidae are represented by Nerita (Heminerita) chilensis [Philippi, 1887]. The new genus Fagnastesia is introduced to represent low-spired trochoideans with a sculpture of nodes below the suture, angulated whorls, and a wide umbilicus. This Miocene Chilean fauna includes genera that have lived at the coast and in shallow, relatively warm water or deeper, much cooler water. This composition therefore suggests that many of the Miocene formations along the central Chilean coast consist of displaced sediments. -

Fish Bulletin 161. California Marine Fish Landings for 1972 and Designated Common Names of Certain Marine Organisms of California
UC San Diego Fish Bulletin Title Fish Bulletin 161. California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California Permalink https://escholarship.org/uc/item/93g734v0 Authors Pinkas, Leo Gates, Doyle E Frey, Herbert W Publication Date 1974 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA THE RESOURCES AGENCY OF CALIFORNIA DEPARTMENT OF FISH AND GAME FISH BULLETIN 161 California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California By Leo Pinkas Marine Resources Region and By Doyle E. Gates and Herbert W. Frey > Marine Resources Region 1974 1 Figure 1. Geographical areas used to summarize California Fisheries statistics. 2 3 1. CALIFORNIA MARINE FISH LANDINGS FOR 1972 LEO PINKAS Marine Resources Region 1.1. INTRODUCTION The protection, propagation, and wise utilization of California's living marine resources (established as common property by statute, Section 1600, Fish and Game Code) is dependent upon the welding of biological, environment- al, economic, and sociological factors. Fundamental to each of these factors, as well as the entire management pro- cess, are harvest records. The California Department of Fish and Game began gathering commercial fisheries land- ing data in 1916. Commercial fish catches were first published in 1929 for the years 1926 and 1927. This report, the 32nd in the landing series, is for the calendar year 1972. It summarizes commercial fishing activities in marine as well as fresh waters and includes the catches of the sportfishing partyboat fleet. Preliminary landing data are published annually in the circular series which also enumerates certain fishery products produced from the catch. -

Guide to the Identification of Larval and Earlyjuvenile Poachers Fronn
NOAA Technical Report NMFS 137 May 1998 Guide to the Identification of Larval and EarlyJuvenile Poachers (Sco~aendfornnes:~onddae) fronn the Northeastern Pacific Ocean and Bering Sea Morgan S. Busby u.s. Department ofCommerce U.S. DEPARTMENT OF COMMERCE WILLIAM M. DALEY NOAA SECRETARY National Oceanic and Atmospheric Administration Technical D.James Baker Under Secretary for Oceans and Atmosphere Reports NMFS National Marine Fisheries Service Technical Reports of the Fishery Bulletin Rolland A. Schmitten Assistant Administrator for Fisheries Scientific Editor Dr.John B. Pearce Northeast Fisheries Science Center National Marine Fisheries Service, NOAA 166 Water Street Woods Hole, Massachusetts 02543-1097 Editorial Conunittee Dr. Andrew E. Dizon National Marine Fisheries Service Dr. Linda L. Jones National Marine Fisheries Service Dr. Richard D. Methot Nauonal Marine Fisheries Service Dr. Theodore W. Pietsch University of Washington Dr. Joseph E. Powers National Marine Fisheries Service Dr. Titn D. Stnith National Marine Fisheries Service Managing Editor Shelley E. Arenas Scientific Publications Office National Marine Fisheries Service, NOAA 7600 Sand Point Way N.E. Seattle, Washington 98115-0070 The NOAA Technical Report NMFS (ISSN 0892-8908) series is published by the Scientific Publications Office, Na tional Marine Fisheries Service, NOAA, 7600 Sand Point Way N.E., Seattle, WA The NOAA Technual Report NMFS series of the Fishery Bulletin carries peer-re 98115-0070. viewed, lengthy original research reports, taxonomic keys, species synopses, flora The Secretary of Commerce has de and fauna studies, and data intensive reports on investigations in fishery science, termined that the publication of this se engineering, and economics. The series was established in 1983 to replace two ries is necessary in the transaction of the subcategories of the Technical Report series: "Special Scientific Report-Fisher public business required by law of tllis ies" and "Circular." Copies of the NOAA Technual Report NMFS are available free Department.