Fcrl5 and T-Bet Define Influenza-Specific Memory B Cells That Predict Long-Lived Antibody 2 Responses 3 4 Anoma Nellore1, Christopher D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Characteristics of B Cell-Associated Gene Expression in Patients With
MOLECULAR MEDICINE REPORTS 13: 4113-4121, 2016 Characteristics of B cell-associated gene expression in patients with coronary artery disease WENWEN YAN*, HAOMING SONG*, JINFA JIANG, WENJUN XU, ZHU GONG, QIANGLIN DUAN, CHUANGRONG LI, YUAN XIE and LEMIN WANG Department of Internal Medicine, Division of Cardiology, Tongji Hospital, Tongji University School of Medicine, Shanghai 200065, P.R. China Received May 19, 2015; Accepted February 12, 2016 DOI: 10.3892/mmr.2016.5029 Abstract. The current study aimed to identify differentially with the two other groups. Additionally the gene expression expressed B cell-associated genes in peripheral blood mono- levels of B cell regulatory genes were measured. In patients nuclear cells and observe the changes in B cell activation at with AMI, CR1, LILRB2, LILRB3 and VAV1 mRNA expres- different stages of coronary artery disease. Groups of patients sion levels were statistically increased, whereas, CS1 and IL4I1 with acute myocardial infarction (AMI) and stable angina (SA), mRNAs were significantly reduced compared with the SA and as well as healthy volunteers, were recruited into the study control groups. There was no statistically significant difference (n=20 per group). Whole human genome microarray analysis in B cell-associated gene expression levels between patients was performed to examine the expression of B cell-associated with SA and the control group. The present study identified the genes among these three groups. The mRNA expression levels downregulation of genes associated with BCRs, B2 cells and of 60 genes associated with B cell activity and regulation were B cell regulators in patients with AMI, indicating a weakened measured using reverse transcription-quantitative polymerase T cell-B cell interaction and reduced B2 cell activation during chain reaction. -
Type of the Paper (Article
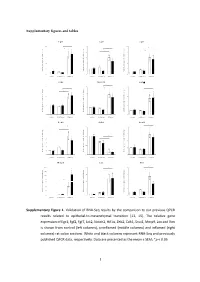
Supplementary figures and tables E g r 1 F g f2 F g f7 1 0 * 5 1 0 * * e e e * g g g * n n n * a a a 8 4 * 8 h h h * c c c d d d * l l l o o o * f f f * n n n o o o 6 3 6 i i i s s s s s s e e e r r r p p p x x x e e e 4 2 4 e e e n n n e e e g g g e e e v v v i i i t t t 2 1 2 a a a l l l e e e R R R 0 0 0 c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d J a k 2 N o tc h 2 H if1 * 3 4 6 * * * e e e g g g n n n a a * * a * h h * h c c c 3 * d d * d l l l * o o o f f 2 f 4 n n n o o o i i i s s s s s s e e e r r 2 r p p p x x x e e e e e e n n n e e 1 e 2 g g g e e 1 e v v v i i i t t t a a a l l l e e e R R R 0 0 0 c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d Z e b 2 C d h 1 S n a i1 * * 7 1 .5 4 * * e e e g g g 6 n n n * a a a * h h h c c c 3 * d d d l l l 5 o o o f f f 1 .0 * n n n * o o o i i i 4 * s s s s s s e e e r r r 2 p p p x x x 3 e e e e e e n n n e e e 0 .5 g g g 2 e e e 1 v v v i i i t t t a a a * l l l e e e 1 * R R R 0 0 .0 0 c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d M m p 9 L o x V im 2 0 0 2 0 8 * * * e e e * g g g 1 5 0 * n n n * a a a * h h h * c c c 1 5 * 6 d d d l l l 1 0 0 o o o f f f n n n o o o i i i 5 0 s s s s s s * e e e r r r 1 0 4 3 0 p p p * x x x e e e * e e e n n n e e e 2 0 g g g e e e 5 2 v v v i i i t t t a a a l l l 1 0 e e e R R R 0 0 0 c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d c o n tro l u n in fla m e d in fla m e d Supplementary Figure 1. -

Monoclonal Antibody-Based Therapy As a New Treatment Strategy in Multiple Myeloma
Leukemia (2012) 26, 199–213 & 2012 Macmillan Publishers Limited All rights reserved 0887-6924/12 www.nature.com/leu REVIEW Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma NWCJ van de Donk1, S Kamps1, T Mutis2 and HM Lokhorst1 1Department of Hematology, University Medical Center Utrecht, Utrecht, The Netherlands and 2Department of Clinical Chemistry and Hematology, University Medical Center Utrecht, Utrecht, The Netherlands The introduction of autologous stem cell transplantation the myeloma patients achieved a partial response (PR) or stable combined with the introduction of immunomodulatory drugs disease following rituximab therapy. All these patients expressed (IMiDs) and proteasome inhibitors has significantly improved CD20 on their myeloma cells.2 However, as only B15–20% of survival of multiple myeloma patients. However, ultimately the majority of patients will develop refractory disease, indicating all myeloma patients express CD20 on their bone marrow the need for new treatment modalities. In preclinical and clinical plasma cells, new targets for immunotherapy need to be studies, promising results have been obtained with several identified. The search for other targets has led to the develop- monoclonal antibodies (mAbs) targeting the myeloma tumor ment of mAbs targeting growth factor receptors or adhesion cell or the bone marrow microenvironment. The mechanisms molecules on myeloma cells. Other newly developed mAbs underlying the therapeutic efficacy of these mAbs include are directed against cellular or non-cellular components of the direct induction of tumor cell apoptosis via inhibition or activation of target molecules, complement-dependent cyto- bone marrow microenvironment, resulting in the neutraliza- toxicity and antibody-dependent cell-mediated cytotoxicity tion of growth factors, inhibition of angiogenesis, modulation (ADCC). -

Supplementary Table 1
Supplementary Table 1. 492 genes are unique to 0 h post-heat timepoint. The name, p-value, fold change, location and family of each gene are indicated. Genes were filtered for an absolute value log2 ration 1.5 and a significance value of p ≤ 0.05. Symbol p-value Log Gene Name Location Family Ratio ABCA13 1.87E-02 3.292 ATP-binding cassette, sub-family unknown transporter A (ABC1), member 13 ABCB1 1.93E-02 −1.819 ATP-binding cassette, sub-family Plasma transporter B (MDR/TAP), member 1 Membrane ABCC3 2.83E-02 2.016 ATP-binding cassette, sub-family Plasma transporter C (CFTR/MRP), member 3 Membrane ABHD6 7.79E-03 −2.717 abhydrolase domain containing 6 Cytoplasm enzyme ACAT1 4.10E-02 3.009 acetyl-CoA acetyltransferase 1 Cytoplasm enzyme ACBD4 2.66E-03 1.722 acyl-CoA binding domain unknown other containing 4 ACSL5 1.86E-02 −2.876 acyl-CoA synthetase long-chain Cytoplasm enzyme family member 5 ADAM23 3.33E-02 −3.008 ADAM metallopeptidase domain Plasma peptidase 23 Membrane ADAM29 5.58E-03 3.463 ADAM metallopeptidase domain Plasma peptidase 29 Membrane ADAMTS17 2.67E-04 3.051 ADAM metallopeptidase with Extracellular other thrombospondin type 1 motif, 17 Space ADCYAP1R1 1.20E-02 1.848 adenylate cyclase activating Plasma G-protein polypeptide 1 (pituitary) receptor Membrane coupled type I receptor ADH6 (includes 4.02E-02 −1.845 alcohol dehydrogenase 6 (class Cytoplasm enzyme EG:130) V) AHSA2 1.54E-04 −1.6 AHA1, activator of heat shock unknown other 90kDa protein ATPase homolog 2 (yeast) AK5 3.32E-02 1.658 adenylate kinase 5 Cytoplasm kinase AK7 -

Mouse and Human Fcr Effector Functions
Pierre Bruhns Mouse and human FcR effector € Friederike Jonsson functions Authors’ addresses Summary: Mouse and human FcRs have been a major focus of Pierre Bruhns1,2, Friederike J€onsson1,2 attention not only of the scientific community, through the cloning 1Unite des Anticorps en Therapie et Pathologie, and characterization of novel receptors, and of the medical commu- Departement d’Immunologie, Institut Pasteur, Paris, nity, through the identification of polymorphisms and linkage to France. disease but also of the pharmaceutical community, through the iden- 2INSERM, U760, Paris, France. tification of FcRs as targets for therapy or engineering of Fc domains for the generation of enhanced therapeutic antibodies. The Correspondence to: availability of knockout mouse lines for every single mouse FcR, of Pierre Bruhns multiple or cell-specific—‘a la carte’—FcR knockouts and the Unite des Anticorps en Therapie et Pathologie increasing generation of hFcR transgenics enable powerful in vivo Departement d’Immunologie approaches for the study of mouse and human FcR biology. Institut Pasteur This review will present the landscape of the current FcR family, 25 rue du Docteur Roux their effector functions and the in vivo models at hand to study 75015 Paris, France them. These in vivo models were recently instrumental in re-defining Tel.: +33145688629 the properties and effector functions of FcRs that had been over- e-mail: [email protected] looked or discarded from previous analyses. A particular focus will be made on the (mis)concepts on the role of high-affinity Acknowledgements IgG receptors in vivo and on results from antibody engineering We thank our colleagues for advice: Ulrich Blank & Renato to enhance or abrogate antibody effector functions mediated by Monteiro (FacultedeMedecine Site X. -

Engineered Type 1 Regulatory T Cells Designed for Clinical Use Kill Primary
ARTICLE Acute Myeloid Leukemia Engineered type 1 regulatory T cells designed Ferrata Storti Foundation for clinical use kill primary pediatric acute myeloid leukemia cells Brandon Cieniewicz,1* Molly Javier Uyeda,1,2* Ping (Pauline) Chen,1 Ece Canan Sayitoglu,1 Jeffrey Mao-Hwa Liu,1 Grazia Andolfi,3 Katharine Greenthal,1 Alice Bertaina,1,4 Silvia Gregori,3 Rosa Bacchetta,1,4 Norman James Lacayo,1 Alma-Martina Cepika1,4# and Maria Grazia Roncarolo1,2,4# Haematologica 2021 Volume 106(10):2588-2597 1Department of Pediatrics, Division of Stem Cell Transplantation and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 2Stanford Institute for Stem Cell Biology and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 3San Raffaele Telethon Institute for Gene Therapy, Milan, Italy and 4Center for Definitive and Curative Medicine, Stanford School of Medicine, Stanford, CA, USA *BC and MJU contributed equally as co-first authors #AMC and MGR contributed equally as co-senior authors ABSTRACT ype 1 regulatory (Tr1) T cells induced by enforced expression of interleukin-10 (LV-10) are being developed as a novel treatment for Tchemotherapy-resistant myeloid leukemias. In vivo, LV-10 cells do not cause graft-versus-host disease while mediating graft-versus-leukemia effect against adult acute myeloid leukemia (AML). Since pediatric AML (pAML) and adult AML are different on a genetic and epigenetic level, we investigate herein whether LV-10 cells also efficiently kill pAML cells. We show that the majority of primary pAML are killed by LV-10 cells, with different levels of sensitivity to killing. Transcriptionally, pAML sensitive to LV-10 killing expressed a myeloid maturation signature. -

Development and Validation of the B Cell-Associated Fc Receptor-Like
Hindawi BioMed Research International Volume 2020, Article ID 8509805, 11 pages https://doi.org/10.1155/2020/8509805 Research Article Development and Validation of the B Cell-Associated Fc Receptor- like Molecule-Based Prognostic Signature in Skin Cutaneous Melanoma Yu Liu,1 Yiding Chen ,2 Xianyu Hu,3 Jialin Meng ,2 and Xiaojing Li 1 1Department of Plastic Surgery, First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China 2Department of Urology, The First Affiliated Hospital of Anhui Medical University; Institute of Urology & Anhui Province Key Laboratory of Genitourinary Diseases, Anhui Medical University, Hefei, Anhui, China 3Department of General Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China Correspondence should be addressed to Jialin Meng; [email protected] and Xiaojing Li; [email protected] Received 21 March 2020; Revised 8 July 2020; Accepted 3 August 2020; Published 24 August 2020 Academic Editor: Adam Reich Copyright © 2020 Yu Liu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Backgroud. Cutaneous melanoma (CM) causes the highest absolute number of deaths among all types of skin cancers; however, the association between Fc receptor- (FcR-) like (FCRL) molecules and CM remains unclear. Methods. 461 patients with CM from The Cancer Genome Atlast- (TCGA-) CM cohort and 290 pateints from the GSE65904 cohort were enrolled. Student’s t-test was used to compare the differences, and Pearson’s correlation coefficient was employed to evaluate associations. The Kaplan-Meier (K-M) survival analysis was used to evaluate overall survival (OS). -

Monoclonal Anti-Human FCRL5/FCRL3-CFS
Monoclonal Anti-human FCRL5/FCRL3-CFS Catalog Number: FAB20871F Lot Number: AAVB01 100 Tests Reagents Provided Background Information Carboxyfluorescein (CFS)-conjugated mouse monoclonal Fc receptor-like 5 (FCRL5), also known as FcRH5 and IRTA2, anti-human FCRL5/FCRL3: Supplied as 25 µg of antibody in 1 mL saline is a member of the Ig superfamily. It shares sequence containing up to 0.5% BSA and 0.1% sodium azide. homology with the classical Fc receptors for Ig and possesses Clone #: 307307 cytoplasmic ITIM and ITAM-like motifs. Secreted variants and a GPI-anchored form of FCRL5 also exist. FCRL5 is Isotype: mouse IgG 2B expressed in naïve and memory B cells and in plasma cells. Reagents Not Provided The gene for FCRL5 is localized to the human chromosome • Flow Cytometry Staining Buffer (Catalog # FC001) or other Iq21 - 23 region, a hotspot for translocation events. BSA-supplemented saline buffer. Flow Cytometry Validation Storage This antibody has been tested for flow cytometry using CD19+ Reagents are stable for twelve months from date of receipt human lymphocytes. when stored in the dark at 2° - 8° C. 1. Cells may be Fc-blocked with 1 µg of human IgG/105 cells Intended Use for 15 minutes at room temperature. Do not wash excess Designed to quantitatively determine the percentage of cells blocking IgG from this reaction. bearing FCRL5/FCRL3 within a population and qualitatively 2. After blocking, 10 µL of conjugated antibody was added to determine the density of FCRL5/FCRL3 on cell surfaces by 1 - 2.5 x 105 cells and incubated for 30 minutes at room flow cytometry. -

Normalized Expression Values *
Normalized expression values 0.0 0.2 0.4 0.6 0.8 1.0 1.2 PLCG1 * T CD5 MP T B TCF7 cDC UBASH3A BCL11B C5AR1 * MP TLR4 CXCR5 B CD79B VPREB3 XCR1 CADM1 BEND5 * ARGHAP22 * cDC CIITA ZBTB46 FLT3 PLEKHA5 Figure S1. qPCR analysis of the core gene expression signature of cDC, MP, B and T cells in the chicken cell suBsets. RNA from cDC, MP, T and B cells of 2 disUnct pools of 4 chicken spleen was subjected to qPCR detecUon of the core gene expression signatures of immune cell subsets established in Fig. 3 and of transcripts from the mouse and human gene subset selected compendia that could not be detected on the array due to defecUve probes, i.e. ARGHAP22, BEND5, C5AR1, and PLCG1 (labeled by a star on the figure). Data are represented as the mean and SD of relave gene expression levels normalized to GAPDH expression and the maximal expression across the cell types was set to 1 (independent experimental duplicates). B cell T cell cDC Monocyte/MP Chicken Human Mouse Figure S2. Unsupervised hierarchical clustering of orthologous immune response genes across chicken, human and mouse reveals globally conserved clusters of lymphoïd- specific and myeloïd- specific genes. Heatmap of cross-normalized expression profiles for immune response genes present on all three species arrays and regulated at least 2 folds across all cell suBsets, including chicken B cells (c_B), T cells (c_CD3), MP (c_MP) and cDC (c_cDC), human B cells (h_B), T cells (h_CD4_T and h_CD8_T), monocyte-derived MP (h_MoMP), peripheral blood mononucleated cell-derived MP (h_PBMC_MP), non-classical monocytes (h_non- classical_MO), classical monocytes (h_classical_MO), BDCA3+ cDC (h_BDCA3), BDCA1+ cDC (h_BDCA1), murine B cells (m_B), T cells (m_CD4_T and m_CD8_T), peritoneal cavity MP (m_PC_MPII-480HI), lung MP (m_LU_MP), non-classical monocytes (m_non-classical_MO), classical monocytes (m_classical_MO), splenic CD8α+ cDC (m_SP_DC1), suBcutaneous lymph node CD8α+ cDC (m_LN_DC1), splenic CD11B+ cDC (m_SP_DC2), suBcutaneous lymph node CD11B+ cDC (m_LN_DC2). -

Supplementary Material
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Ann Rheum Dis SUPPLEMENTARY MATERIAL SUPPLEMENTARY NOTE: a. Members of the Japanese Research Committee on Idiopathic Osteonecrosis of the Femoral Head -------------------------------------------------------------------------------P2 b. Acknowledgement ------------------------------------------------------------------------------P3 c. Web resources -----------------------------------------------------------------------------------P5 SUPPLEMENTARY FIGURES: Supplementary Figure 1: Quantile-quantile (QQ) plot of the meta-analysis association P values for all variants (left) and the variants outside of known SLE loci (right). ---------------------------------------------------------------------------------------------------P6 Supplementary Figure 2: Regional association plots for the 113 SLE genomic loci. ------------------------------------------------------------------------------------------------------------P7 SUPPLEMENTARY TABLES: Supplementary Table 1: Characteristics of the eight studies used in this meta- analysis-------------------------------------------------------------------------------------------------P28 Supplementary Table 2: Association results for the lead variants within the 113 genomic loci-------------------------------------------------------------------------------------------P29 Supplementary Table 3: Comparison of