Classical Biological Control of the Glassy-Winged Sharpshooter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sharpshooter X Wave: Correlation of an Electrical Penetration Graph Waveform with Xylem Penetration Supports a Hypothesized Mech
ARTHROPODS IN RELATION TO PLANT DISEASES Sharpshooter X Wave: Correlation of an Electrical Penetration Graph Waveform With Xylem Penetration Supports a Hypothesized Mechanism for Xylella fastidiosa Inoculation ELAINE A. BACKUS,1 WENDY J. HOLMES,2 FRED SCHREIBER,2 BRENDON J. REARDON,3,4 3 AND GREGORY P. WALKER Ann. Entomol. Soc. Am. 102(5): 847Ð867 (2009) ABSTRACT Electrical penetration graph (EPG) monitoring is the most rigorous means of obser- vation and quantiÞcation of feeding by piercingÐsucking arthropods. Previous EPG studies with aphids and leafhoppers have demonstrated that the X wave identiÞes when the stylets of these phloem ßuid-ingesting insects make contact with their preferred plant vascular cell, phloem sieve elements. This article presents the Þrst direct evidence of an X wave identifying ingestion from a xylem tracheary element by a xylem ßuid-ingesting type of leafhopper Homalodisca liturata Ball (Hemiptera: Cicadel- lidae: Cicadellinae), whose waveforms are nearly identical to those of the glassy-winged sharpshooter, Homalodisca vitripennis (Germar). We document consistent association of the sharpshooter X wave with salivary sheath termini in xylem, especially ligniÞed secondary xylem cells, and absence of the X wave in the rare instance of ingestion from a nonxylem cell. The sharpshooter X wave is a complex, multicomponent waveform, composed of X wave-speciÞc variants of waveform subtypes B1w (rep- resenting salivation), B1s (representing precibarial valve movement for tasting), types C1 (a new waveform type that may represent egestion) and C2 (a new designation for the waveform type representing ingestion/cibarial pumping). It is proposed that the sharpshooter X wave represents a blended suite of behaviors that function to 1) physically seal stylet tips into the cell via sheath salivation, 2) repeatedly taste then eject (egest) chemical constituents of the cell to determine acceptability, and 3) mechanically test the strength of the stylet seal via trial cibarial pumping (ingestion). -

Répartition De La Population En Polynésie Française En 2017
Répartition de la population en Polynésie française en 2017 PIRAE ARUE Paopao Teavaro Hatiheu PAPEETE Papetoai A r c h MAHINA i p e l d FAA'A HITIAA O TE RA e s NUKU HIVA M a UA HUKA r q PUNAAUIA u HIVA OA i TAIARAPU-EST UA POU s Taiohae Taipivai e PAEA TA HUATA s NUKU HIVA Haapiti Afareaitu FATU HIVA Atuona PAPARA TEVA I UTA MOO REA TAIARAPU-OUEST A r c h i p e l d Puamau TAHITI e s T MANIHI u a HIVA OA Hipu RA NGIROA m Iripau TA KAROA PUKA P UKA o NA PUKA Hakahau Faaaha t u Tapuamu d e l a S o c i é MAKEMO FANGATA U - p e l t é h i BORA BORA G c a Haamene r MAUPITI Ruutia A TA HA A ARUTUA m HUAHINE FAKARAVA b TATAKOTO i Niua Vaitoare RAIATEA e TAHITI r TAHAA ANAA RE AO Hakamaii MOORE A - HIK UE RU Fare Maeva MAIAO UA POU Faie HA O NUKUTAVAKE Fitii Apataki Tefarerii Maroe TUREIA Haapu Parea RIMATARA RURUTU A r c h Arutua HUAHINE i p e TUBUAI l d e s GAMBIE R Faanui Anau RA IVAVAE A u s Kaukura t r Nombre a l AR UTUA d'individus e s Taahuaia Moerai Mataura Nunue 20 000 Mataiva RA PA BOR A B OR A 10 000 Avera Tikehau 7 000 Rangiroa Hauti 3 500 Mahu Makatea 1 000 RURUT U TUBUAI RANGIROA ´ 0 110 Km So u r c e : Re c en se m en t d e la p o p u la ti o n 2 0 1 7 - IS P F -I N SE E Répartition de la population aux Îles Du Vent en 2017 TAHITI MAHINA Paopao Papetoai ARUE PAPEETE PIRAE HITIAA O TE RA FAAA Teavaro Tiarei Mahaena Haapiti PUNAAUIA Afareaitu Hitiaa Papenoo MOOREA 0 2 Km Faaone PAEA Papeari TAIARAPU-EST Mataiea Afaahiti Pueu Toahotu Nombre PAPARA d'individus TEVA I UTA Tautira 20 000 Vairao 15 000 13 000 Teahupoo 10 000 TAIARAPU-OUEST -

Melanaphis Sacchari), in Grain Sorghum
DEVELOPMENT OF A RESEARCH-BASED, USER- FRIENDLY, RAPID SCOUTING PROCEDURE FOR THE INVASIVE SUGARCANE APHID (MELANAPHIS SACCHARI), IN GRAIN SORGHUM By JESSICA CARRIE LINDENMAYER Bachelor of Science in Soil and Crop Sciences Bachelor of Science in Horticulture Colorado State University Fort Collins, Colorado 2013 Master of Science in Entomology and Plant Pathology Oklahoma State University Stillwater, Oklahoma 2015 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY May, 2019 DEVELOPMENT OF A RESEARCH-BASED, USER- FRIENDLY, RAPID SCOUTING PROCEDURE FOR THE INVASIVE SUGARCANE APHID (MELANAPHIS SACCHARI), IN GRAIN SORGHUM Dissertation Approved: Tom A. Royer Dissertation Adviser Kristopher L. Giles Norman C. Elliott Mark E. Payton ii ACKNOWLEDGEMENTS I would like to thank my amazing committee and all my friends and family for their endless support during my graduate career. I would like to say a special thank you to my husband Brad for supporting me in every way one possibly can, I couldn’t have pursued this dream without you. I’d also like to thank my first child, due in a month. The thought of getting to be your mama pushed me to finish strong so you would be proud of me. Lastly, I want to thank my step father Jasper H. Davis III for showing me how to have a warrior’s spirit and to never give up on something, or someone you love. Your love, spirit, and motivational words will always be heard in my heart even while you’re gone. -
![Ooctonus Vulgatus[I] (Hymenoptera, Mymaridae), A](https://docslib.b-cdn.net/cover/8567/ooctonus-vulgatus-i-hymenoptera-mymaridae-a-168567.webp)
Ooctonus Vulgatus[I] (Hymenoptera, Mymaridae), A
A peer-reviewed version of this preprint was published in PeerJ on 24 March 2020. View the peer-reviewed version (peerj.com/articles/8591), which is the preferred citable publication unless you specifically need to cite this preprint. Mesmin X, Chartois M, Genson G, Rossi J, Cruaud A, Rasplus J. 2020. Ooctonus vulgatus (Hymenoptera, Mymaridae), a potential biocontrol agent to reduce populations of Philaenus spumarius (Hemiptera, Aphrophoridae) the main vector of Xylella fastidiosa in Europe. PeerJ 8:e8591 https://doi.org/10.7717/peerj.8591 Ooctonus vulgatus (Hymenoptera, Mymaridae), a potential biocontrol agent to reduce populations of Philaenus spumarius (Hemiptera, Aphrophoridae) the main vector of Xylella fastidiosa in Europe Xavier Mesmin 1, 2 , Marguerite Chartois 2 , Guenaelle Genson 2 , Jean-Pierre Rossi 2 , Astrid Cruaud 2 , Jean-Yves Rasplus Corresp. 2 1 AGAP, INRA, CIRAD, Montpellier SupAgro, Univ Montpellier, INRA, San Giuliano, France 2 CBGP, INRA, CIRAD, IRD, Montpellier SupAgro, Univ Montpellier, Montpellier, France Corresponding Author: Jean-Yves Rasplus Email address: [email protected] As vector of Xylella fastidiosa (Wells, 1987) in Europe, the meadow spittlebug, Philaenus spumarius (Linnaeus, 1758) (Hemiptera: Aphrophoridae) is a species of major concern. Therefore, tools and agents to control this ubiquitous insect that develops and feeds on hundreds of plant species are wanted. We conducted a field survey of P. spumarius eggs in Corsica and provide a first report of Ooctonus vulgatus Haliday, 1833 (Hymenoptera, Mymaridae) as a potential biocontrol agent of P. spumarius in Europe. To allow species identification, we summarized the main characters distinguishing O. vulgatus from other European species of Ooctonus and generated COI DNA barcodes. -

Bulletin D'information
BULLETIN D’INFORMATION #40 60 personnes ont été dépistées positives sur les 2965 tests effectués depuis le début de l’épidémie, en Polynésie française. A ce jour, 4 personnes sont toujours sous surveillance et 56 ont été autorisées à sortir d’isolement. A noter : dorénavant les mises à jour du carré épidémiologique ne se feront qu’en jours ouvrés. Chiffres clés Continuité territoriale Nombre total de cas COVID-19 60 Ce dimanche 10 mai, des résidents polynésiens et Nombre total de cas COVID-19 hospitalisés leurs familles ont pu être rapatriés de métropole au fenua. Au total, ce sont 53 personnes qui sont - en cours 1 logés au centre d’hébergement étudiant (CHE) de - depuis le début de l’épidémie 4 Outumaoro et à Tibériade, à Taiarapu Ouest (cf : photo 1). COVIDNombre-19 de en cas Polynésie COVID-19 français admis ene réanimation 0 Conformément à l’engagement sur l’honneur qu’ils Nombre de décès lié au COVID-19 0 ont signé avant leur départ ils seront placés en quarantaine pendant quatorze jours, à l’issue desquels ils subiront à nouveau un test de Fig. 1. Nombre de cas cumulés COVID-19 en PF par date dépistage qui, s’il est négatif, leur permettra de rentrer à leur domicile. Les personnes qui sont revenues au fenua par le vol de continuité territoriale du 22 avril 2020, ont pu rejoindre leur famille et leur maison, tous ont été testé négatifs, au terme de leur quarantaine. Ce quatrième vol de la continuité territoriale a acheminé près de 15 tonnes de fret pour un volume de 120 mètres cube dont 42 mètres cube, de matériel médical. -

Notes on Neotropical Proconiini (Hemiptera: Cicadellidae: Cicadellinae), IV: Lectotype Designations of Aulacizes Amyot &
Arthropod Systematics & Phylogeny 105 64 (1) 105–111 © Museum für Tierkunde Dresden, ISSN 1863-7221, 30.10.2006 Notes on Neotropical Proconiini (Hemiptera: Cicadellidae: Cicadellinae), IV: lectotype designations of Aulacizes Amyot & Audinet-Serville species described by Germar and revalidation of A. erythrocephala (Germar, 1821) GABRIEL MEJDALANI 1, DANIELA M. TAKIYA 2 & RACHEL A. CARVALHO 1 1 Departamento de Entomologia, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, São Cristóvão, 20940-040 Rio de Janeiro, RJ, Brazil [[email protected]] 2 Center for Biodiversity, Illinois Natural History Survey, 1816 S. Oak Street, Champaign, IL 61820, USA [[email protected]] Received 17.iii.2005, accepted 22.viii.2006. Available online at www.arthropod-systematics.de > Abstract Lectotypes are designated for the sharpshooter species Aulacizes erythrocephala (Germar, 1821) and A. quadripunctata (Germar, 1821) based on recently located specimens from the Germar collection. The former species is reinstated from synonymy of the latter one and is redescribed and illustrated based on specimens from Southeastern Brazil. The male and female genitalia are described for the fi rst time. The two species are similar morphologically, but they can be easily distinguished from each other, as well as from the other species of the genus, by their color patterns. > Key words Membracoidea, Aulacizes quadripunctata, leafhopper, sharpshooter, taxonomy, morphology, Brazil. 1. Introduction This is the fourth paper of a series on the taxonomy redescribed and illustrated. One sharpshooter type of the leafhopper tribe Proconiini in the Neotropical located in the Germar collection (Homalodisca vitri- region. The fi rst three papers included descriptions of pennis (Germar, Year 1821)) was previously designated two new species and notes on other species in the tribe by TAKIYA et al. -

Egg-Laying and Brochosome Production Observed in Glassy-Winged Sharps Hooter
Egg-laying and brochosome production observed in glassy-winged sharps hooter Raymond L. Hix Glassy-winged sharpshooter effective integrated pest management these spots weren’t merely ornaments, (G WSS) females form white spots (IPM) programs, especially aspects re- but he wasn’t sure as to their origin. He on the forewings from secretions lated to insect monitoring. We briefly supposed them to be transferred to the of ultramicroscopic bodies known discuss what is known about GWSS forewings by the hind tibia from the as brochosomes. This occurs af- egg-laying behavior, and present stud- anus. The powdering of the egg mass ter mating of the G WSS and just ies from my laboratory. The implica- was believed to camouflage the eggs prior to egg laying. The first pub- tions of wing-spot formation and from predators and parasites. lished reports of wing spots were brochosome secretions are discussed The makeup, origin and function of made by Riley and Howard in in the context of IPM programs. All white spots in certain leafhoppers 1893. The behaviors associated GWSS brochosome secretions are ei- ther grayish translucent or opaque with brochosome formation could white in comparison to the clear excre- have important implications for in- ment often referred to as “hopper tegrated pest management (IPM) rain.” programs to control G WSS, an im- portant vector of the bacterium Historical perspective that causes Pierce’s disease in Before the turn of the 20th century, grapevines and other crops. Riley and Howard (1893) dispatched Nathan Banks and F.W. Mally to ince 1997, wineries near Temecula Shreveport, La., to investigate prob- Shave lost 20% to 30% of their vines lems in cotton with the GWSS, re- to Pierce‘s disease, which is caused by ferred to locally as a ”sharpshooter” the bacterium Xylellafustidiosa Wells attack. -

Winged Sharpshooter
REPRODUCTIVE AND DEVELOPMENTAL BIOLOGY OF GONATOCERUS ASHMEADI, AN EGG PARASITOID OF THE GLASSY-WINGED SHARPSHOOTER Project leader: Cooperator: Mark Hoddle Leigh Pilkington Dept. of Entomology Dept. of Entomology University of California University of California Riverside, CA 92521 Riverside, CA 92521 Reporting period: The results reported here are from work conducted from April 2004 to October 2004. ABSTRACT The reproductive and developmental biology of Gonatocerus ashmeadi Girault, a self-introduced parasitoid of the glassy- winged sharpshooter (GWSS) Homalodisca coagulata Say, was determined at five constant temperatures in the laboratory; 15; 20; 25; 30; and 33°C. Wasps at each experimental temperature were given, on average, between 10 and 15 GWSS eggs per day for its natural life for oviposition. At 30°C, immature G. ashmeadi sustained the highest mortality rates as adult emergence was lowest at this temperature. The largest proportion of female offspring was produced at 25°C and lifetime fecundity was greatest at 25°C. The development time was greatest at 15°C and lowest at 30°C. Mean adult longevity was inversely related to temperature with a maximum of approximately 30 days at 15°C to a minimum of approximately two days at 33°C. INTRODUCTION The mymarid wasp species Gonatocerus ashmeadi Girault, G. triguttatus Girault, G. morrilli Howard, and G. fasciatus Girault are the most common natural enemies associated with the insect pest Homalodisca coagulata in it’s home range of southeastern USA and northeastern Mexico (Triapitsyn and Phillips, 2000). The wasp G. ashmeadi is a self-introduced resident of California and most likely came into the state in parasitized Homalodisca coagulata eggs (Vickerman et al., 2004) and has established widely in association with H. -
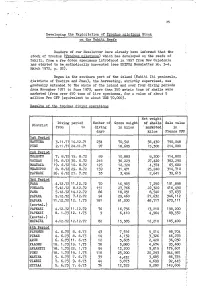
Developing the Exploitation of <I>Trochus Niloticus</I> Stock on the Tahiti Reefs
35 Developing the Exploitation of Trochu3 niloticus Stock onithe_Ta'hiti Reefs Readers of our Newsletter have already been informed that the stock of trochus (Trochug niloticus) which has developed on the reefs of Tahiti, from a few dozen specimens introduced in 1957 from Hew Caledonia has started to be methodically harvested (see SPIPDA Newsletter ITo. 3-4, March 1972, p. 32). Begun in the southern part of the island (Tahiti Iti peninsula, districts of Tautira and Pueu), the harvesting, strictly supervised, was gradually extended to the whole of the island and over four diving periods fjrom November 1971 to June 1973? more than 350 metric tons of shells were marketed (from over 450 tons of live specimens, for a value of about 5 million Frs CTP (equivalent to about US$ 70,000). Results of .the trochus. diving operations Net weight Diving period Number of Gross weight of shells Sale value District from to diving in kilos ' marketed in days kilos Francs CPP 1st Period TAUTIRA 3-11.71 I4.l2.7i 234 70,541 56,430 790,048 .. PUEU 2.11 .71 24.11.71 97 18,605 ... 15,300 214,000 2nd Period TOAHOTU 7. 8.72 15. 8,72 89 10r883 8,200 114,800 : VAIRAO 15. 8.72 30. 8.72 216 36J229 27,420 362,290 , MAATAIA. 19. 6.72 10. 8o72 125 12,724 4,374 65,600 TEAHUPOO 8. 8.72 29. 8.72 159 31,471 25,240' 314,710 PAPEARI 26. 6.72 27. 7.72 35 3,456 2,641 39,615 ; 3rd Period FAAA 4.12,72 11.12.72 70 16,983 7,250 101,898 PIMAAUA 5.12.72 8,12.72 151 27,766 .22,320 416,490 PAEA 5.12.72 14*12.72 86 18,051 8, 540 97,633 PAPARA 5.12.72 7.12.72 94 29,460 21,632 346,112 PAPARA 11.12.72 12. -

Glassy-Winged Sharpshooter & Pierce's Disease Research
Glassy-winged Sharpshooter & Pierce’s Disease Research Summaries FY 00-01/01-02 Glassy-winged Sharpshooter and Pierce’s Disease Research Summaries FY 2000-2001 and FY 2001-2002 Funding Agencies: Almond Board of California (Almond) American Vineyard Foundation (AVF) California Citrus Nursery Advisory Board (CA Citrus Nu rsery) California Competitive Grant Program for Research in Viticulture/Enology (CCGPRVE) California Department of Food and Agriculture (CDFA) California Department of Food and Agriculture – Assembly Bill 1232 (CDFA AB 1232) California Department of Transportation (Cal Trans) California Raisin Marketing Board (Raisin) California Table Grape Commission (Table) Citrus Research Board (Citrus Board) City of Temecula (Temecula) County of Riverside (Riverside) Kern/Tulare GWSS Task Force (Kersn/Tulare) UC Pierce’s Disease Grant Program (UC - PD) UC Pierce’s Disease Integrated Pest Management Program (UC - IPM) USDA - Agricultural Research Service (USDA-ARS) USDA - Animal Plant Health Inspection Service (USDA-APHIS) USDA – Community Credit Corporation (USDA-CCC) USDA – Cooperative State Research Education & Extension Service (USDA-CSREES) Viticulture Consortium (VC) Published by: California Department of Food and Agriculture December 2001 Summaries compiled by Patrick Gleeson Cover Design: Jay Van Rein To order additional copies of this publication, contact: M. Athar Tariq California Department of Food and Agriculture Pierce’s Disease Control Program 2014 Capitol Avenue, Suite 109 Sacramento, CA 95814 Telephone: (916) 322-2804 Fax: (916) 322-3924 http://www.cdfa.ca.gov/phpps/pdcp E-mail: [email protected] Printing: Copeland Printing Sacramento, CA Note: The summaries in this publication have not been subject to independent scientific review. The California Department of Food and Agriculture makes no warranty, expressed or implied, and assumes no legal liability for the information in this publication. -

Progeny Quality of Gonatocerus Ashmeadi (Hymenoptera: Mymaridae) Reared on Stored Eggs of Homalodisca Coagulata (Hemiptera: Cicadellidae)
BIOLOGICAL AND MICROBIAL CONTROL Progeny Quality of Gonatocerus ashmeadi (Hymenoptera: Mymaridae) Reared on Stored Eggs of Homalodisca coagulata (Hemiptera: Cicadellidae) 1 2 WEN-LONG CHEN AND ROGER A. LEOPOLD J. Econ. Entomol. 100(3): 685Ð694 (2007) ABSTRACT This study assessed the effects of refrigerated storage on the suitability of eggs of the glassy-winged sharpshooter, Homalodisca coagulata (Say) (Hemiptera: Cicadellidae), as hosts for propagation of the parasitoid Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae). Develop- ment of the host eggs was terminated by chilling at 2ЊC for 5 d before storage was initiated at 10ЊC for up to 70 d. Parasitism, adult emergence rate, developmental time, and sex ratio were used to gauge the suitability of the eggs as hosts after storage. In addition to these measures, demographic growth parameters also were used to assess the quality of the wasp progeny through the F2 generation. Host eggs stored 20 d remained fully acceptable to the wasps for attack. Although the parasitism rate decreased with storage time, Ͼ 80% adult parasitoid emergence was realized from eggs stored 30 d. After 70 d storage, adult emergence rate was decreased by 48%, fecundity decreased by 53%, female production by 19%, developmental time was extended 3 d, and female longevity was shortened 5 d. The emergence pattern of F1 but not F2 adults varied with storage time of the parental and grand- parental hosts, respectively. For the F2 generation, emergence rate, development, and sex ratio did not vary with storage time when the F1 parents parasitized fresh host eggs. Demographic parameters Ͼ for the F1 population showed that net reproductive rate was 20 although it decreased signiÞcantly after their parental host eggs were stored for Ͼ 30 d. -

FUNGI ASSOCIATED with the GLASSY-WINGED SHARPSHOOTER, Homalodisca Coagulata, in ITS NATIVE RANGE
FUNGI ASSOCIATED WITH THE GLASSY-WINGED SHARPSHOOTER, Homalodisca coagulata, IN ITS NATIVE RANGE By S. ELIE BREAUX A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2005 Copyright 2005 by S. Elie Breaux This document is dedicated to Stefanie, always there. ACKNOWLEDGMENTS I would like to thank the members of my committee for their support, perseverance, and knowledge. I consider myself lucky to have found in them the willingness to take a chance on a student. I would like to thank Dr. Linda Young for extensive assistance in the statistical analysis portion of this study. I would also like to thank my family. My father has always been a student of nature. Raised with his love of the outdoors, the choice to take this path was made without reservation. My mother has always provided every kind of support a son could ask for, free of expectation or judgment. I thank Nicholas and Silas for being so entertaining. They are so different in nature, but time spent with either of them makes one realize what is important. And finally, I would like to thank Stefanie. Always generous with encouragement and unwavering in support, there is no way I could have done this without her. iv TABLE OF CONTENTS page ACKNOWLEDGMENTS ................................................................................................. iv LIST OF TABLES...........................................................................................................