The Impact of the Kelvin Redefinition and Recent Primary Thermometry on Temperature Measurements for Meteorology and Climatology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Units and Conversions
Units and Conversions This unit of the Metrology Fundamentals series was developed by the Mitutoyo Institute of Metrology, the educational department within Mitutoyo America Corporation. The Mitutoyo Institute of Metrology provides educational courses and free on-demand resources across a wide variety of measurement related topics including basic inspection techniques, principles of dimensional metrology, calibration methods, and GD&T. For more information on the educational opportunities available from Mitutoyo America Corporation, visit us at www.mitutoyo.com/education. This technical bulletin addresses an important aspect of the language of measurement – the units used when reporting or discussing measured values. The dimensioning and tolerancing practices used on engineering drawings and related product specifications use either decimal inch (in) or millimeter (mm) units. Dimensional measurements are therefore usually reported in either of these units, but there are a number of variations and conversions that must be understood. Measurement accuracy, equipment specifications, measured deviations, and errors are typically very small numbers, and therefore a more practical spoken language of units has grown out of manufacturing and precision measurement practice. Metric System In the metric system (SI or International System of Units), the fundamental unit of length is the meter (m). Engineering drawings and measurement systems use the millimeter (mm), which is one thousandths of a meter (1 mm = 0.001 m). In general practice, however, the common spoken unit is the “micron”, which is slang for the micrometer (m), one millionth of a meter (1 m = 0.001 mm = 0.000001 m). In more rare cases, the nanometer (nm) is used, which is one billionth of a meter. -

Consultative Committee for Amount of Substance; Metrology in Chemistry and Biology CCQM Working Group on Isotope Ratios (IRWG) S
Consultative Committee for Amount of Substance; Metrology in Chemistry and Biology CCQM Working Group on Isotope Ratios (IRWG) Strategy for Rolling Programme Development (2021-2030) 1. EXECUTIVE SUMMARY In April 2017, the Consultative Committee for Amount of Substance; Metrology in Chemistry and Biology (CCQM) established a task group to study the metrological state of isotope ratio measurements and to formulate recommendations to the Consultative Committee (CC) regarding potential engagement in this field. In April 2018 the Isotope Ratio Working Group (IRWG) was established by the CCQM based on the recommendation of the task group. The main focus of the IRWG is on the stable isotope ratio measurement science activities needed to improve measurement comparability to advance the science of isotope ratio measurement among National Metrology Institutes (NMIs) and Designated Institutes (DIs) focused on serving stake holder isotope ratio measurement needs. During the current five-year mandate, it is expected that the IRWG will make significant advances in: i. delta scale definition, ii. measurement comparability of relative isotope ratio measurements, iii. comparable measurement capabilities for C and N isotope ratio measurement; and iv. the understanding of calibration modalities used in metal isotope ratio characterization. 2. SCIENTIFIC, ECONOMIC AND SOCIAL CHALLENGES Isotopes have long been recognized as markers for a wide variety of molecular processes. Indeed, applications where isotope ratios are used provide scientific, economic, and social value. Early applications of isotope measurements were recognized with the 1943 Nobel Prize for Chemistry which included the determination of the water content in the human body, determination of solubility of various low-solubility salts, and development of the isotope dilution method which has since become the cornerstone of analytical chemistry. -

Lord Kelvin and the Age of the Earth.Pdf
ME201/MTH281/ME400/CHE400 Lord Kelvin and the Age of the Earth Lord Kelvin (1824 - 1907) 1. About Lord Kelvin Lord Kelvin was born William Thomson in Belfast Ireland in 1824. He attended Glasgow University from the age of 10, and later took his BA at Cambridge. He was appointed Professor of Natural Philosophy at Glasgow in 1846, a position he retained the rest of his life. He worked on a broad range of topics in physics, including thermody- namics, electricity and magnetism, hydrodynamics, atomic physics, and earth science. He also had a strong interest in practical problems, and in 1866 he was knighted for his work on the transtlantic cable. In 1892 he became Baron Kelvin, and this name survives as the name of the absolute temperature scale which he proposed in 1848. During his long career, Kelvin published more than 600 papers. He was elected to the Royal Society in 1851, and served as president of that organization from 1890 to 1895. The information in this section and the picture above were taken from a very useful web site called the MacTu- tor History of Mathematics Archive, sponsored by St. Andrews University. The web address is http://www-history.mcs.st-and.ac.uk/~history/ 2 kelvin.nb 2. The Age of the Earth The earth shows it age in many ways. Some techniques for estimating this age require us to observe the present state of a time-dependent process, and from that observation infer the time at which the process started. If we believe that the process started when the earth was formed, we get an estimate of the earth's age. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Role of Metrology in Conformity Assessment
Role of Metrology in Conformity Assessment Andy Henson Director of International Liaison and Communications of the BIPM Metrology, the science of measurement… and its applications “When you can measure what you are speaking about, and express it in numbers, you know something about it; but when you cannot express it in numbers, your knowledge is of a meagre and unsatisfactory kind” William Thompson (Lord Kelvin): Lecture on "Electrical Units of Measurement" (3 May 1883) Conformity assessment Overall umbrella of measures taken by : ‐manufacturers ‐customers ‐regulatory authorities ‐independent third parties To assess that a product/service meets standards or technical regulations 2 Metrology is a part of our lives from birth Best regulation Safe food Technical evidence Safe treatment Safe baby food Manufacturing Safe traveling Globalization weighing of baby Healthcare Innovation Climate change Without metrology, you can’t discover, design, build, test, manufacture, maintain, prove, buy or operate anything safely and reliably. From filling your car with petrol to having an X‐ray at a hospital, your life is surrounded by measurements. In industry, from the thread of a nut and bolt and the precision machined parts on engines down to tiny structures on micro and nano components, all require an accurate measurement that is recognized around the world. Good measurement allows country to remain competitive, trade throughout the world and improve quality of life. www.bipm.org 3 Metrology, the science of measurement As we have seen, measurement science is not purely the preserve of scientists. It is something of vital importance to us all. The intricate but invisible network of services, suppliers and communications upon which we are all dependent rely on metrology for their efficient and reliable operation. -
Maths Objectives – Measurement
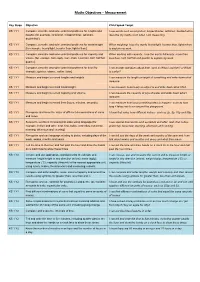
Maths Objectives – Measurement Key Stage Objective Child Speak Target KS 1 Y1 Compare, describe and solve practical problems for lengths and I use words such as long/short, longer/shorter, tall/short, double/half to heights [for example, long/short, longer/shorter, tall/short, describe my maths work when I am measuring. double/half]. KS 1 Y1 Compare, describe and solve practical problems for mass/weight When weighing, I use the words heavy/light, heavier than, lighter than [for example, heavy/light, heavier than, lighter than]. to explain my work. KS 1 Y1 Compare, describe and solve practical problems for capacity and When working with capacity, I use the words full/empty, more than, volume [for example, full/empty, more than, less than, half, half full, less than, half, half full and quarter to explain my work. quarter]. KS 1 Y1 Compare, describe and solve practical problems for time [for I can answer questions about time, such as Who is quicker? or What example, quicker, slower, earlier, later]. is earlier? KS 1 Y1 Measure and begin to record lengths and heights. I can measure the length or height of something and write down what measure. KS 1 Y1 Measure and begin to record mass/weight. I can measure how heavy an object is and write down what I find. KS 1 Y1 Measure and begin to record capacity and volume. I can measure the capacity of jugs of water and write down what I measure. KS 1 Y1 Measure and begin to record time (hours, minutes, seconds). I can measure how long something takes to happen - such as how long it takes me to run around the playground. -

UNITS This Appendix Explains Some of the Abbreviations1•2 Used For
APPENDIX: UNITS This appendix explains some of the abbreviations1•2 used for units in this book and gives conversion factors to SI units and atomic units: length 1 a0 = 1 bohr = 0.5291771 X 10-10 m 1 A= 1 Angstrom= lo-10 m = 1.889727 ao mass 1 me = 1 atomic unit of mass = mass of an electron 9.109534 X 10-31 kg = 5.485803 X 10-4 U 1 u 1 universal atomic mass unit = one twelfth the mass of a 12c atom 1.6605655 x lo-27 kg = 1822.887 me time 1 t Eh 1 = 1 atomic unit of time = 2.418884 x l0-17 s 1 s = 1 second = 4.134137 x 1016 t/Eh temperature 1 K = 1 Kelvin amount of substance 1 mol = 1 mole 6.022045 x 1023 atoms, molecules, or formula units energy 1 cm-1 = 1 wavenumber 1 kayser 1.986477 x lo-23 J 4.556335 x 10-6 Eh 857 858 APPENDIX: UNITS 1 kcal/mol = 1 kilocalorie per mole 4.184 kJ/mol = 1.593601 x 10-3 Eh 1 eV 1 electron volt = 1.602189 x lo-19 J 3.674902 X 10-2 Eh 1 Eh 1 hartree = 4.359814 x lo-18 J Since so many different energy units are used in the book, it is helpful to have a conversion table. Such a table was calculated from the recommended values of Cohen and Taylor3 for the physical censtants and is given in Table 1. REFERENCES 1. "Standard for Metric Practice", American Society for Testing and Materials, Philadelphia (1976). -

Standard Caption Abberaviation
TECHNICAL SHEET Page 1 of 2 STANDARD CAPTION ABBREVIATIONS Ref: T120 – Rev 10 – March 02 The abbreviated captions listed are used on all instruments except those made to ANSI C39. 1-19. Captions for special scales to customers’ requirements must comply with BS EN 60051, unless otherwise specified at time of ordering. * DENOTES captions applied at no extra cost. Other captions on request. ELECTRICAL UNITS UNIT SYMBOL UNIT SYMBOL Direct Current dc Watt W * Alternating current ac Milliwatts mW * Amps A * Kilowatts kW * Microamps µA * Megawatts MW * Milliamps mA * Vars VAr * Kiloamps kA * Kilovars kVAr * Millivolts mV * Voltamperes VA * Kilovolts kV * Kilovoltamperes kVA * Cycles Hz * Megavoltamperes MVA * Power factor cos∅ * Ohms Ω * Synchroscope SYNCHROSCOPE * Siemens S Micromhos µmho MECHANICAL UNITS Inches in Micrometre (micron) µm Square inches in2 Millimetre mm Cubic inches in3 Square millimetres mm2 Inches per second in/s * Cubic millimetres mm3 Inches per minute in/min * Millimetres per second mm/s * Inches per hour in/h * Millimetres per minute mm/min * Inches of mercury in hg Millimetres per hour mm/h * Feet ft Millimetres of mercury mm Hg Square feet ft2 Centimetre cm Cubic feet ft3 Square centimetres cm2 Feet per second ft/s * Cubic centimetres cm3 Feet per minute ft/min * Cubic centimetres per min cm3/min Feet per hour ft/h * Centimetres per second cm/s * Foot pound ft lb Centimetres per minute cm/min * Foot pound force ft lbf Centimetres per hour cm/h * Hours h Decimetre dm Yards yd Square decimetre dm2 Square yards yd2 Cubic -

The Kelvin and Temperature Measurements
Volume 106, Number 1, January–February 2001 Journal of Research of the National Institute of Standards and Technology [J. Res. Natl. Inst. Stand. Technol. 106, 105–149 (2001)] The Kelvin and Temperature Measurements Volume 106 Number 1 January–February 2001 B. W. Mangum, G. T. Furukawa, The International Temperature Scale of are available to the thermometry commu- K. G. Kreider, C. W. Meyer, D. C. 1990 (ITS-90) is defined from 0.65 K nity are described. Part II of the paper Ripple, G. F. Strouse, W. L. Tew, upwards to the highest temperature measur- describes the realization of temperature able by spectral radiation thermometry, above 1234.93 K for which the ITS-90 is M. R. Moldover, B. Carol Johnson, the radiation thermometry being based on defined in terms of the calibration of spec- H. W. Yoon, C. E. Gibson, and the Planck radiation law. When it was troradiometers using reference blackbody R. D. Saunders developed, the ITS-90 represented thermo- sources that are at the temperature of the dynamic temperatures as closely as pos- equilibrium liquid-solid phase transition National Institute of Standards and sible. Part I of this paper describes the real- of pure silver, gold, or copper. The realiza- Technology, ization of contact thermometry up to tion of temperature from absolute spec- 1234.93 K, the temperature range in which tral or total radiometry over the tempera- Gaithersburg, MD 20899-0001 the ITS-90 is defined in terms of calibra- ture range from about 60 K to 3000 K is [email protected] tion of thermometers at 15 fixed points and also described. -

What Is Temperature
City Research Online City, University of London Institutional Repository Citation: Kyriacou, P. A. (2010). Temperature sensor technology. In: Jones, D. P. (Ed.), Biomedical Sensors. (pp. 1-38). New York: Momentum Press. ISBN 9781606500569 This is the accepted version of the paper. This version of the publication may differ from the final published version. Permanent repository link: https://openaccess.city.ac.uk/id/eprint/3539/ Link to published version: Copyright: City Research Online aims to make research outputs of City, University of London available to a wider audience. Copyright and Moral Rights remain with the author(s) and/or copyright holders. URLs from City Research Online may be freely distributed and linked to. Reuse: Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. City Research Online: http://openaccess.city.ac.uk/ [email protected] Biomedical Sensors: Temperature Sensor Technology P A Kyriacou, PhD Reader in Biomedical Engineering School of Engineering and Mathematical Sciences City University, London EC1V 0HB “...the clinical thermometer ranks in importance with the stethoscope. A doctor without his thermometer is like a sailor without his compass” Family Physician, 1882 Celsius thermometer (attached to a barometer) made by J.G. Hasselström, Stockholm, late 18th century Page 1 of 39 1. Introduction Human body temperature is of vital importance to the well being of the person and therefore it is routinely monitored to indicate the state of the person’s health. -

Handbook of MASS MEASUREMENT
Handbook of MASS MEASUREMENT FRANK E. JONES RANDALL M. SCHOONOVER CRC PRESS Boca Raton London New York Washington, D.C. © 2002 by CRC Press LLC Front cover drawing is used with the consent of the Egyptian National Institute for Standards, Gina, Egypt. Back cover art from II Codice Atlantico di Leonardo da Vinci nella Biblioteca Ambrosiana di Milano, Editore Milano Hoepli 1894–1904. With permission from the Museo Nazionale della Scienza e della Tecnologia Leonardo da Vinci Milano. Library of Congress Cataloging-in-Publication Data Jones, Frank E. Handbook of mass measurement / Frank E. Jones, Randall M. Schoonover p. cm. Includes bibliographical references and index. ISBN 0-8493-2531-5 (alk. paper) 1. Mass (Physics)—Measurement. 2. Mensuration. I. Schoonover, Randall M. II. Title. QC106 .J66 2002 531’.14’0287—dc21 2002017486 CIP This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage or retrieval system, without prior permission in writing from the publisher. The consent of CRC Press LLC does not extend to copying for general distribution, for promotion, for creating new works, or for resale. Specific permission must be obtained in writing from CRC Press LLC for such copying. -

The Kibble Balance and the Kilogram
C. R. Physique 20 (2019) 55–63 Contents lists available at ScienceDirect Comptes Rendus Physique www.sciencedirect.com The new International System of Units / Le nouveau Système international d’unités The Kibble balance and the kilogram La balance de Kibble et le kilogramme ∗ Stephan Schlamminger , Darine Haddad NIST, 100 Bureau Drive, Gaithersburg, MD 20899, USA a r t i c l e i n f o a b s t r a c t Article history: Dr. Bryan Kibble invented the watt balance in 1975 to improve the realization of the unit Available online 25 March 2019 for electrical current, the ampere. With the discovery of the Quantum Hall effect in 1980 by Dr. Klaus von Klitzing and in conjunction with the previously predicted Josephson effect, Keywords: this mechanical apparatus could be used to measure the Planck constant h. Following a Unit of mass proposal by Quinn, Mills, Williams, Taylor, and Mohr, the Kibble balance can be used to Kilogram Planck constant realize the unit of mass, the kilogram, by fixing the numerical value of Planck’s constant. Kibble balance In 2017, the watt balance was renamed to the Kibble balance to honor the inventor, who Revised SI passed in 2016. This article explains the Kibble balance, its role in the redefinition of the Josephson effect unit of mass, and attempts an outlook of the future. Quantum Hall effect Published by Elsevier Masson SAS on behalf of Académie des sciences. This is an open access article under the CC BY-NC-ND license Mots-clés : (http://creativecommons.org/licenses/by-nc-nd/4.0/).