Species Status Assessment Report for the Round Hickorynut Mussel (Obovaria Subrotunda)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Relationships with Large Wood in Small Streams
Amencan F~sheriesSociety Symposium 37:179-193, 2003 Fish Relationships with Large Wood in Small Streams USDA Forest Service, Southern Research Station, Department ofFisheries and Wildlife Virginia Tech, Blacksburg, Virginia 24060, USA USDA Forest Service, Southern Research Station 1000 Front Street, Oxford, Massachusetts 38655, USA Abstracf.-Many ecological processes are associated with large wood in streams, such as forming habitat critical for fish and a host of other organisms. Wood loading in streams varies with age and species of riparian vegetation, stream size, time since last disturbance, and history of land use. Changes in the landscape resulting from homesteading, agriculture, and logging have altered forest environments, which, in turn, changed the physical and biological characteristics of many streams worldwide. Wood is also important in creating refugia for fish and other aquatic species. Removing wood from streams typically results in loss of pool habitat and overall complexity as well as fewer and smaller individuals of both coldwater and warmwater fish species. The life histories of more than 85 species of fish have some association with large wood for cover, spawning (egg attachment, nest materials), and feeding. Many other aquatic organisms, such as crayfish, certain species of freshwater mus- sels, and turtles, also depend on large wood during at least part of their life cycles. Introduction Because decay rate and probability of displace- ment are a function of size, large pieces have a Large wood can profoundly influence the struc- greater influence on habitat and physical processes ture and function of aquatic habitats from head- than small pieces. In general, rootwads, branches, waters to estuaries. -
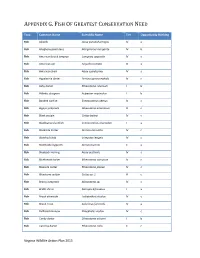
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -

Stability, Persistence and Habitat Associations of the Pearl Darter Percina Aurora in the Pascagoula River System, Southeastern USA
Vol. 36: 99–109, 2018 ENDANGERED SPECIES RESEARCH Published June 13 https://doi.org/10.3354/esr00897 Endang Species Res OPENPEN ACCESSCCESS Stability, persistence and habitat associations of the pearl darter Percina aurora in the Pascagoula River System, southeastern USA Scott R. Clark1,4,*, William T. Slack2, Brian R. Kreiser1, Jacob F. Schaefer1, Mark A. Dugo3 1Department of Biological Sciences, The University of Southern Mississippi, Hattiesburg, Mississippi 39406, USA 2US Army Engineer Research and Development Center, Environmental Laboratory EEA, Vicksburg, Mississippi 39180, USA 3Mississippi Valley State University, Department of Natural Sciences and Environmental Health, Itta Bena, Mississippi 38941, USA 4Present address: Department of Biology and Museum of Southwestern Biology, University of New Mexico, Albuquerque, New Mexico 87131, USA ABSTRACT: The southeastern United States represents one of the richest collections of aquatic biodiversity worldwide; however, many of these taxa are under an increasing threat of imperil- ment, local extirpation, or extinction. The pearl darter Percina aurora is a small-bodied freshwater fish endemic to the Pearl and Pascagoula river systems of Mississippi and Louisiana (USA). The last collected specimen from the Pearl River drainage was taken in 1973, and it now appears that populations in this system are likely extirpated. This reduced the historical range of this species by approximately 50%, ultimately resulting in federal protection under the US Endangered Species Act in 2017. To better understand the current distribution and general biology of extant popula- tions, we analyzed data collected from a series of surveys conducted in the Pascagoula River drainage from 2000 to 2016. Pearl darters were captured at relatively low abundance (2.4 ± 4.0 ind. -

Age Influences Resistance to Infestation by Freshwater Pearl Mussel (Margaritifera Margaritifera)Glochidia
Parasitology Research (2019) 118:1519–1532 https://doi.org/10.1007/s00436-019-06300-2 IMMUNOLOGY AND HOST-PARASITE INTERACTIONS - ORIGINAL PAPER Host (Salmo trutta) age influences resistance to infestation by freshwater pearl mussel (Margaritifera margaritifera)glochidia Janhavi Marwaha1 & Hans Aase2 & Juergen Geist3 & Bernhard C. Stoeckle3 & Ralph Kuehn4,5 & Per Johan Jakobsen1 Received: 30 October 2018 /Accepted: 20 March 2019 /Published online: 1 April 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The freshwater pearl mussel (Margaritifera margaritifera) is an endangered bivalve with an obligate parasitic stage on salmonids. Host suitability studies have shown that glochidial growth and load vary significantly between host strains as well as among individuals of a suitable strain. Variation in host suitability has been linked to environmental conditions, host age and/or size, genetic composition of the host and parasite, or a combination of these factors. In our study, we wanted to investigate if brown trout (Salmo trutta) displayed an age-dependent response to glochidial infestation. We hypothesised that 1+ naive brown trout hosts tolerate glochidial infestation better than 0+ hosts. In order to test our hypothesis, we infested 0+ and 1+ hatchery reared brown trout with glochidia from closely related mothers and kept them under common garden conditions. This allowed us to observe a pure age dependent host response to infestation, as we eliminated the confounding effect of genotype-specific host interactions. We analysed the interaction between glochidial load and host condition, weight and length, and observed a signif- icant age-dependent relationship. Glochidial load was negatively correlated to host condition in 0+ fish hosts and positively correlated in 1+ hosts. -

Louisiana's Animal Species of Greatest Conservation Need (SGCN)
Louisiana's Animal Species of Greatest Conservation Need (SGCN) ‐ Rare, Threatened, and Endangered Animals ‐ 2020 MOLLUSKS Common Name Scientific Name G‐Rank S‐Rank Federal Status State Status Mucket Actinonaias ligamentina G5 S1 Rayed Creekshell Anodontoides radiatus G3 S2 Western Fanshell Cyprogenia aberti G2G3Q SH Butterfly Ellipsaria lineolata G4G5 S1 Elephant‐ear Elliptio crassidens G5 S3 Spike Elliptio dilatata G5 S2S3 Texas Pigtoe Fusconaia askewi G2G3 S3 Ebonyshell Fusconaia ebena G4G5 S3 Round Pearlshell Glebula rotundata G4G5 S4 Pink Mucket Lampsilis abrupta G2 S1 Endangered Endangered Plain Pocketbook Lampsilis cardium G5 S1 Southern Pocketbook Lampsilis ornata G5 S3 Sandbank Pocketbook Lampsilis satura G2 S2 Fatmucket Lampsilis siliquoidea G5 S2 White Heelsplitter Lasmigona complanata G5 S1 Black Sandshell Ligumia recta G4G5 S1 Louisiana Pearlshell Margaritifera hembeli G1 S1 Threatened Threatened Southern Hickorynut Obovaria jacksoniana G2 S1S2 Hickorynut Obovaria olivaria G4 S1 Alabama Hickorynut Obovaria unicolor G3 S1 Mississippi Pigtoe Pleurobema beadleianum G3 S2 Louisiana Pigtoe Pleurobema riddellii G1G2 S1S2 Pyramid Pigtoe Pleurobema rubrum G2G3 S2 Texas Heelsplitter Potamilus amphichaenus G1G2 SH Fat Pocketbook Potamilus capax G2 S1 Endangered Endangered Inflated Heelsplitter Potamilus inflatus G1G2Q S1 Threatened Threatened Ouachita Kidneyshell Ptychobranchus occidentalis G3G4 S1 Rabbitsfoot Quadrula cylindrica G3G4 S1 Threatened Threatened Monkeyface Quadrula metanevra G4 S1 Southern Creekmussel Strophitus subvexus -

REPORT FOR: Preliminary Analysis for Identification, Distribution, And
REPORT FOR: Preliminary Analysis for Identification, Distribution, and Conservation Status of Species of Fusconaia and Pleurobema in Arkansas Principle Investigators: Alan D. Christian Department of Biological Sciences, Arkansas State University, P.O. Box 599, State University, Arkansas 72467; [email protected]; Phone: (870)972-3082; Fax: (870)972-2638 John L. Harris Department of Biological Sciences, Arkansas State University, P.O. Box 599, State University, Arkansas 72467 Jeanne Serb Department of Ecology, Evolution, and Organismal Biology, Iowa State University, 251 Bessey Hall, Ames, Iowa 50011 Graduate Research Assistant: David M. Hayes, Department of Environmental Science, P.O. Box 847, State University, Arkansas 72467: [email protected] Kentaro Inoue, Department of Environmental Science, P.O. Box 847, State University, Arkansas 72467: [email protected] Submitted to: William R. Posey Malacologist and Commercial Fisheries Biologist, AGFC P.O. Box 6740 Perrytown, Arkansas 71801 April 2008 EXECUTIVE SUMMARY There are currently 13 species of Fusconaia and 32 species of Pleurobema recognized in the United States and Canada. Twelve species of Pleurobema and two species of Fusconaia are listed as Threatened or Endangered. There are 75 recognized species of Unionidae in Arkansas; however this number may be much higher due to the presence of cryptic species, many which may reside within the Fusconaia /Pleurobema complex. Currently, three species of Fusconaia and three species of Pleurobema are recognized from Arkansas. The true conservation status of species within these genera cannot be determined until the taxonomic identity of populations is confirmed. The purpose of this study was to begin preliminary analysis of the species composition of Fusconaia and Pleurobema in Arkansas and to determine the phylogeographic relationships within these genera through mitochondrial DNA sequencing and conchological analysis. -

Freshwater Mussel Survey of Clinchport, Clinch River, Virginia: Augmentation Monitoring Site: 2006
Freshwater Mussel Survey of Clinchport, Clinch River, Virginia: Augmentation Monitoring Site: 2006 By: Nathan L. Eckert, Joe J. Ferraro, Michael J. Pinder, and Brian T. Watson Virginia Department of Game and Inland Fisheries Wildlife Diversity Division October 28th, 2008 Table of Contents Introduction....................................................................................................................... 4 Objective ............................................................................................................................ 5 Study Area ......................................................................................................................... 6 Methods.............................................................................................................................. 6 Results .............................................................................................................................. 10 Semi-quantitative .................................................................................................. 10 Quantitative........................................................................................................... 11 Qualitative............................................................................................................. 12 Incidental............................................................................................................... 12 Discussion........................................................................................................................ -

“A People Who Have Not the Pride to Record Their History Will Not Long
STATE HISTORIC PRESERVATION OFFICE i “A people who have not the pride to record their History will not long have virtues to make History worth recording; and Introduction no people who At the rear of Old Main at Bethany College, the sun shines through are indifferent an arcade. This passageway is filled with students today, just as it was more than a hundred years ago, as shown in a c.1885 photograph. to their past During my several visits to this college, I have lingered here enjoying the light and the student activity. It reminds me that we are part of the past need hope to as well as today. People can connect to historic resources through their make their character and setting as well as the stories they tell and the memories they make. future great.” The National Register of Historic Places recognizes historic re- sources such as Old Main. In 2000, the State Historic Preservation Office Virgil A. Lewis, first published Historic West Virginia which provided brief descriptions noted historian of our state’s National Register listings. This second edition adds approx- Mason County, imately 265 new listings, including the Huntington home of Civil Rights West Virginia activist Memphis Tennessee Garrison, the New River Gorge Bridge, Camp Caesar in Webster County, Fort Mill Ridge in Hampshire County, the Ananias Pitsenbarger Farm in Pendleton County and the Nuttallburg Coal Mining Complex in Fayette County. Each reveals the richness of our past and celebrates the stories and accomplishments of our citizens. I hope you enjoy and learn from Historic West Virginia. -

Indian Warfare, Household Competency, and the Settlement of the Western Virginia Frontier, 1749 to 1794
Graduate Theses, Dissertations, and Problem Reports 2007 Indian warfare, household competency, and the settlement of the western Virginia frontier, 1749 to 1794 John M. Boback West Virginia University Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Boback, John M., "Indian warfare, household competency, and the settlement of the western Virginia frontier, 1749 to 1794" (2007). Graduate Theses, Dissertations, and Problem Reports. 2566. https://researchrepository.wvu.edu/etd/2566 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Indian Warfare, Household Competency, and the Settlement of the Western Virginia Frontier, 1749 to 1794 John M. Boback Dissertation submitted to the College of Arts and Sciences at West Virginia University in partial fulfillment of the requirements for the degree of Doctor -

Summary Tables of Waterbody Conditions, List of Prioritized Impaired Waters, and Monitoring and TMDL Schedules
Section Ohio 2012 Integrated Report L Summary Tables of Waterbody Conditions, List of Prioritized Impaired Waters, and Monitoring and TMDL Schedules Section L contains tables showing the 303(d) listing details for each of the assessment unit types: Section L1: Status of Watershed Assessment Units Section L2: Status of Large River Assessment Units Section L3: Status of Lake Erie Assessment Units Section L4: Section 303(d) List of Prioritized Impaired Waters (Category 5) Section L5: Monitoring and TMDL Schedules for Ohio’s Watershed and Large River Assessment Units Section L6: Category 4B Demonstrations Contained in Approved Ohio TMDLs to Date In Sections L1 through L5, there are four columns labeled, in order, “Human Health,” “Recreation,” “Aquatic Life” and “PDW Supply.” These four columns represent each beneficial use included in the 303(d) list of impaired waters, and the numbers in the columns represent the category for that assessment unit for that beneficial use. The categories are defined below. Category definitions for the 2012 Integrated Report and 303(d) list Category1 Subcategory 0 No waters currently utilized for water supply 1 Use attaining h Historical data t TMDL complete; AU is now attaining water quality standards x Retained from 2008 IR 2 Not applicable in Ohio system 3 Use attainment unknown h Historical data i Insufficient data t TMDL complete; included in TMDL(s) for other units, but there may be no or not enough data to assess this unit x Retained from 2008 IR 4 Impaired; TMDL not needed A TMDL complete B Other required control measures will result in attainment of use C Not a pollutant h Historical data n Natural causes and sources x Retained from 2008 IR 5 Impaired; TMDL needed M Mercury h Historical data x Retained from 2008 IR 1 Shading indicates categories defined by U.S. -

Scaleshell Mussel Recovery Plan
U.S. Fish and Wildlife Service Scaleshell Mussel Recovery Plan (Leptodea leptodon) February 2010 Department of the Interior United States Fish and Wildlife Service Great Lakes – Big Rivers Region (Region 3) Fort Snelling, MN Cover photo: Female scaleshell mussel (Leptodea leptodon), taken by Dr. M.C. Barnhart, Missouri State University Disclaimer This is the final scaleshell mussel (Leptodea leptodon) recovery plan. Recovery plans delineate reasonable actions believed required to recover and/or protect listed species. Plans are published by the U.S. Fish and Wildlife Service and sometimes prepared with the assistance of recovery teams, contractors, state agencies, and others. Objectives will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Recovery plans do not necessarily represent the views or the official positions or approval of any individuals or agencies involved in plan formulation, other than the U.S. Fish and Wildlife Service. They represent the official position of the U.S. Fish and Wildlife Service only after being signed by the Regional Director. Approved recovery plans are subject to modifications as dictated by new findings, changes in species status, and the completion of recovery actions. The plan will be revised as necessary, when more information on the species, its life history ecology, and management requirements are obtained. Literature citation: U.S. Fish and Wildlife Service. 2010. Scaleshell Mussel Recovery Plan (Leptodea leptodon). U.S. Fish and Wildlife Service, Fort Snelling, Minnesota. 118 pp. Recovery plans can be downloaded from the FWS website: http://endangered.fws.gov i ACKNOWLEDGMENTS Many individuals and organizations have contributed to our knowledge of the scaleshell mussel and work cooperatively to recover the species. -

Candy Darter Recovery Outline
U.S. Fish & Wildlife Service Candy Darter Recovery Outline Photo by Corey Dunn, University of Missouri Species Name: Candy Darter (Etheostoma osburni) Species Range: Upper Kanawha River Basin including the Gauley, Greenbrier, and New River Watersheds including portions of Greenbrier, Pocahontas, Nicholas, and Webster counties in West Virginia; and Bland, Giles, and Wythe counties, Virginia. The range of the species is shown in figure 1 below. Recovery Priority Number: 5; explanation provided below Listing Status: Endangered; November 21, 2019; 83 FR 58747 Lead Regional Office/Cooperating RO(s): Northeast Region, Hadley MA Lead Field Office/Cooperating FO(s): West Virginia Field Office, Elkins WV; Southwestern Virginia Field Office, Abingdon, VA; White Sulphur Springs National Fish Hatchery, White Sulphur Springs, WV Lead Contact: Barbara Douglas, 304-636-6586 ext 19. [email protected] 1) Background This section provides a brief overview of the ecology and conservation of the candy darter. This information is more fully described in the Species Status Assessment (SSA), the final listing rule, and the proposed critical habitat rule. These documents are available at: https://www.fws.gov/northeast/candydarter/ 1 Type and Quality of Available Information to Date: Important Information Gaps and Treatment of Uncertainties One of the primary threats that resulted in the listing of the candy darter under the Endangered Species Act (ESA) is the spread of the introduced variegate darter (Etheostoma variatum). This species hybridizes with the candy darter. Key information gaps and areas of uncertainty for the recovery and management of the candy darter include whether there are any habitat or other natural factors that could limit the spread of variegate darter.