1 Arsenic and Other Water-Quality Issues Affecting Groundwater, Indus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annexure D: Thar Coal Analysis
American Journal of Scientific Research 160 ISSN 1450-223X Issue 11(2010), pp.92-102 Annexure-'D' © EuroJournals Publishing, Inc. 2010 http://www.eurojournals.com/ajsr.htm Composition, Trace Element Contents and Major Ash Constituents of Thar Coal, Pakistan M. Afzal Farooq Choudry Department of Environmental Science, FUUAST, Karachi, Pakistan E-mail: [email protected] Yasmin Nurgis Environmental Research Center, Bahria University, Karachi, Pakistan E-mail: [email protected] Mughal Sharif Environmental Research Center, Bahria University, Karachi, Pakistan E-mail: [email protected] Amjad Ali Mahmood Geological Survey of Pakistan, Karachi, Pakistan Haq Nawaz Abbasi Department of Environmental Science, FUUAST, Karachi, Pakistan E-mail: [email protected] Abstract Thar coalfield is a part of the Thar Desert of Pakistan. Pakistan has coal reserves of 185 billion tons, of this Thar coal reserves account for 175 billion tons spread over a single geographically contained area of 9100 sq km in the south eastern part of the Sindh. It is bounded in the north, east and south by India, in the west by the irrigated Indus river flood plain. The terrain is sandy and rough with sand dunes forming the topography. Various physio-chemical parameters including chemical composition of coal ashes, distribution of trace elements in them, were analyzed to understand the coal prospects and its share in the domestic energy production. In addition a preliminary study have also undertaken on the factors that effect the chemical composition of coal ashes. The apparent rank is high volatile Lignite “B” coal. Arithmetic mean values for proximate analysis of coals (as received basis; n=54) show these coals to be 6.83% Ash, 29.55% volatile matter, 19.2% fixed carbon and 44.3% moisture and have a heat of combustion of 6094 BTU/lb. -

"Family Motacillidae" with Reference to Pakistan
Journal of Bioresource Management Volume 2 Issue 3 Article 10 Short Report: Description and Distribution of Wagtails "Family Motacillidae" with Reference to Pakistan Nadia Yousuf Bioresource Research Centre, Isalamabad, Pakistan Kainaat William Bioresource Research Centre, Islamabad, Pakistan Madeeha Manzoor Bioresource Research Centre, Islamabad, Pakistan, [email protected] Balqees Khanum Bioresource Research Centre, Islamabad, Pakistan Follow this and additional works at: https://corescholar.libraries.wright.edu/jbm Part of the Biodiversity Commons, and the Biology Commons Recommended Citation Yousuf, N., William, K., Manzoor, M., & Khanum, B. (2015). Short Report: Description and Distribution of Wagtails "Family Motacillidae" with Reference to Pakistan, Journal of Bioresource Management, 2 (3). DOI: 10.35691/JBM.5102.0034 ISSN: 2309-3854 online This Article is brought to you for free and open access by CORE Scholar. It has been accepted for inclusion in Journal of Bioresource Management by an authorized editor of CORE Scholar. For more information, please contact [email protected]. Short Report: Description and Distribution of Wagtails "Family Motacillidae" with Reference to Pakistan © Copyrights of all the papers published in Journal of Bioresource Management are with its publisher, Center for Bioresource Research (CBR) Islamabad, Pakistan. This permits anyone to copy, redistribute, remix, transmit and adapt the work for non-commercial purposes provided the original work and source is appropriately cited. Journal -

Health Facilities in Thatta- Sindh Province
PAKISTAN: Health facilities in Thatta- Sindh province Matiari Balochistan Type of health facilities "D District headquarter (DHQ) Janghari Tando "T "B Tehsil headquarter (THQ) Allah "H Civil hospital (CH) Hyderabad Yar "R Rural health center (RHC) "B Basic health unit (BHU) Jamshoro "D Civil dispensary (CD) Tando Las Bela Hafiz Road Shah "B Primary Boohar Muhammad Ramzan Secondary "B Khan Haijab Tertiary Malkhani "D "D Karachi Jhirck "R International Boundary MURTAZABAD Tando City "B Jhimpir "B Muhammad Province Boundary Thatta Pir Bux "D Brohi Khan District Boundary Khair Bux Muhammad Teshil Boundary Hylia Leghari"B Pinyal Jokhio Jungshahi "D "B "R Chatto Water Bodies Goth Mungar "B Jokhio Chand Khan Palijo "B "B River "B Noor Arbab Abdul Dhabeeji Muhammad Town Hai Palejo Thatta Gharo Thaheem "D Thatta D "R B B "B "H " "D Map Doc Name: PAK843_Thatta_hfs_L_A3_ "" Gujjo Thatta Shah Ashabi Achar v1_20190307 Town "B Jakhhro Creation Date: 07 March 2019 Badin Projection/Datum: GCS/WGS84 Nominal Scale at A3 paper size: 1:690,000 Haji Ghulammullah Pir Jo "B Muhammad "B Goth Sodho RAIS ABDUL 0 10 20 30 "B GHANI BAGHIAR Var "B "T Mirpur "R kms ± Bathoro Sindh Map data source(s): Mirpur GAUL, PCO, Logistic Cluster, OCHA. Buhara Sakro "B Disclaimers: The designations employed and the presentation of material on this map do not imply the expression of any Thatta opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Vehari, 17/1/1970 Matric 12/07/2014 Younis Ismil Punjab
Renewal List S/NO REN# / NAME FATHER'S NAME PRESENT ADDRESS DATE OF ACADEMIC REN DATE BIRTH QUALIFICATION 1 25303 MUHAMMAD MUHAMMAD CHAK NO. 33/WB, LUDDEN ROAD , VEHARI, 17/1/1970 MATRIC 12/07/2014 YOUNIS ISMIL PUNJAB 2 25586 MUHAMMAD ALAM ABDUL HAMEED CHAK NO. 297 E.B. TEH, BUREWALA DISTT. 14-12- MATRIC 13/07/2014 VEHARI , VEHARI, PUNJAB 1980 3 21990 MUHAMMAD MOHABBAT ALI CHAK NO. 136/EB TEH, BUREWALA DISTT,, 10-10- MATRIC 14/07/2014 SHAFIQ VEHARI, PUNJAB 1980 4 25937 SABA SHAMS QAZI SHAMS UD- VEHARI MAIN ROAD H NO. 231/N MOH, SHARKI 6-7-1983 FA 14/07/2014 DIN COLONY, VEHARI, PUNJAB 5 21418 BASHARAT ALI MUHAMMAD H.NO.27-2 HOUSING SCHEMEBUREWALA, 25/2/1979 MATRIC 14/07/2014 SULEMAN VEHARI, PUNJAB AKHTAR 6 21531 MUHAMMAD RANA USMAN CHAK NO.9/WB, VEHARI, PUNJAB 9/2/1973 MATRIC 14/07/2014 SUBHAN ALI KHAN 7 21508 MUHAMMAD M. AKRAM HASHMI CHOWK NEAR KHANEWAL CHOWK 20/7/1974 MATRIC 14/07/2014 SALEEM 11/W.B, VEHARI, PUNJAB 8 21508 SARDAR SHABIR DILAWAR HOUSE NO. 85/B VEHARI ROAD VEHARI , VEHARI, 2-2-1984 MATRIC 15/07/2014 AKBAR HUSSIAN PUNJAB 9 28179 RABIA GHULAM H/NO. 6679 /-B-1 WARD NO. 2 MOH, RASOOL 15-4-1984 MATRIC 15/07/2014 HUSSAIN PURA MAILSI DISTT, , VEHARI, PUNJAB 10 26928 FAHAD BASHIR ZAHID NAVEED H/NO. 114/CMIAN MARKEET SHARQICOLONY , 12-3-1984 MATRIC 15/07/2014 PASHA VEHARI, PUNJAB 11 30186 MUHAMMAD ALLAH DAWAYA BASTI GHULAM SINDHI P/O FATEH PUR TEH, 15-8-1984 MATRIC 16/07/2014 ZAWAR HUSSAIN MELSI DISTT, VEHARI , VEHARI, PUNJAB 12 33502 MUHAMMAD MUHAMMAD ALI MOH, RIAZ ABAD MULTAN ROAD MELSI DISTT, 3-1-1972 MATRIC 16/7/2014 ASLAM VEHARI , VEHARI, PUNJAB 13 39879 GHULAM ABDUL REHMAN GALI/ MOH, CHAH KUMAR WALA HALEEMKHACHI 1-2-1982 MATRIC 4/8/2014 MURTAZA TEH, MAILSI DISTT,, VEHARI, PUNJAB 14 31433 SABIRA BEGUM MIRZA SHER ALI H NO. -

Ghfbooksouthasia.Pdf
1000 BC 500 BC AD 500 AD 1000 AD 1500 AD 2000 TAXILA Pakistan SANCHI India AJANTA CAVES India PATAN DARBAR SQUARE Nepal SIGIRIYA Sri Lanka POLONNARUWA Sri Lanka NAKO TEMPLES India JAISALMER FORT India KONARAK SUN TEMPLE India HAMPI India THATTA Pakistan UCH MONUMENT COMPLEX Pakistan AGRA FORT India SOUTH ASIA INDIA AND THE OTHER COUNTRIES OF SOUTH ASIA — PAKISTAN, SRI LANKA, BANGLADESH, NEPAL, BHUTAN —HAVE WITNESSED SOME OF THE LONGEST CONTINUOUS CIVILIZATIONS ON THE PLANET. BY THE END OF THE FOURTH CENTURY BC, THE FIRST MAJOR CONSOLIDATED CIVILIZA- TION EMERGED IN INDIA LED BY THE MAURYAN EMPIRE WHICH NEARLY ENCOMPASSED THE ENTIRE SUBCONTINENT. LATER KINGDOMS OF CHERAS, CHOLAS AND PANDYAS SAW THE RISE OF THE FIRST URBAN CENTERS. THE GUPTA KINGDOM BEGAN THE RICH DEVELOPMENT OF BUILT HERITAGE AND THE FIRST MAJOR TEMPLES INCLUDING THE SACRED STUPA AT SANCHI AND EARLY TEMPLES AT LADH KHAN. UNTIL COLONIAL TIMES, ROYAL PATRONAGE OF THE HINDU CULTURE CONSTRUCTED HUNDREDS OF MAJOR MONUMENTS INCLUDING THE IMPRESSIVE ELLORA CAVES, THE KONARAK SUN TEMPLE, AND THE MAGNIFICENT CITY AND TEMPLES OF THE GHF-SUPPORTED HAMPI WORLD HERITAGE SITE. PAKISTAN SHARES IN THE RICH HISTORY OF THE REGION WITH A WEALTH OF CULTURAL DEVELOPMENT AROUND ISLAM, INCLUDING ADVANCED MOSQUE ARCHITECTURE. GHF’S CONSER- VATION OF ASIF KHAN TOMB OF THE JAHANGIR COMPLEX IN LAHORE, PAKISTAN WILL HELP PRESERVE A STUNNING EXAMPLE OF THE GLORIOUS MOGHUL CIVILIZATION WHICH WAS ONCE CENTERED THERE. IN THE MORE REMOTE AREAS OF THE REGION, BHUTAN, SRI LANKA AND NEPAL EACH DEVELOPED A UNIQUE MONUMENTAL FORM OF WORSHIP FOR HINDUISM. THE MOST CHALLENGING ASPECT OF CONSERVATION IS THE PLETHORA OF HERITAGE SITES AND THE LACK OF RESOURCES TO COVER THE COSTS OF CONSERVATION. -

Solutions for Energy Crisis in Pakistan I
Solutions for Energy Crisis in Pakistan i ii Solutions for Energy Crisis in Pakistan Solutions for Energy Crisis in Pakistan iii ACKNOWLEDGEMENTS This volume is based on papers presented at the two-day national conference on the topical and vital theme of Solutions for Energy Crisis in Pakistan held on May 15-16, 2013 at Islamabad Hotel, Islamabad. The Conference was jointly organised by the Islamabad Policy Research Institute (IPRI) and the Hanns Seidel Foundation, (HSF) Islamabad. The organisers of the Conference are especially thankful to Mr. Kristof W. Duwaerts, Country Representative, HSF, Islamabad, for his co-operation and sharing the financial expense of the Conference. For the papers presented in this volume, we are grateful to all participants, as well as the chairpersons of the different sessions, who took time out from their busy schedules to preside over the proceedings. We are also thankful to the scholars, students and professionals who accepted our invitation to participate in the Conference. All members of IPRI staff — Amjad Saleem, Shazad Ahmad, Noreen Hameed, Shazia Khurshid, and Muhammad Iqbal — worked as a team to make this Conference a success. Saira Rehman, Assistant Editor, IPRI did well as stage secretary. All efforts were made to make the Conference as productive and result oriented as possible. However, if there were areas left wanting in some respect the Conference management owns responsibility for that. iv Solutions for Energy Crisis in Pakistan ACRONYMS ADB Asian Development Bank Bcf Billion Cubic Feet BCMA -

Power Project at Keti Bundar
INFORMATION MEMORANDUM 2X660 MW IMPORTED/THAR COAL POWER PROJECTS AT KETI BANDER SINDH COAL AUTHORITY ENERGY DEPARTMENT GOVERNMENT OF SINDH Bungalow No.16 E Street, Zamzama Park, DHA Phase-V, Karachi. Phone: 99251507 1 THE LAND AND THE GOVERNMENT Pakistan, a land of many splendors and opportunities, the repository of a unique blend of history and culture from the East and the west, the cradle of one of the oldest civilizations which developed around the Indus Valley. It is the ninth most popular country of the world with 132.35 million tough, conscientious, hard working people, wishing and striving hard to enter into the 21st century as equal partners in the community of developed nations. It is located between 23 and 37 degrees latitude north and 61 and 76 degrees longitude east. Flanked by Iran and land- locked Afghanistan in the west and the Central Asian Republics and China in the north, Pakistan can rightly boost of having a significant location advantages with a vast only partially tapped market of 200 million people. The affluent Gulf States are just across the Arabian Sea to the south and provide an additional opportunity of a high consumption market. The geographical location, with one of the highest peaks of the world in the north and vast plains in the south, offers an unusual diversity of temperatures ranging from sub-zero levels on the mountains in winter to scorching heat in the plains in summer, providing friendly habitat to exquisite range of flora and fauna and a large variety of agricultural crops used for both foods and raw material for industries. -

Pdf | 951.36 Kb
P a g e | 1 Operation Updates Report Pakistan: Monsoon Floods DREF n° MDRPK019 GLIDE n° FL-2020-000185-PAK Operation update n° 1; Date of issue: 6/10/2020 Timeframe covered by this update: 10/08/2020 – 07/09/2020 Operation start date: 10/08/2020 Operation timeframe: 6 months; End date: 28/02/2021 Funding requirements (CHF): DREF second allocation amount CHF 339,183 (Initial DREF CHF 259,466 - Total DREF budget CHF 598,649) N° of people being assisted: 96,250 (revised from the initially planned 68,250 people) Red Cross Red Crescent Movement partners currently actively involved in the operation: IFRC Pakistan Country Office is actively involved in the coordination and is supporting Pakistan Red Crescent Society (PRCS) in this operation. In addition, PRCS is maintaining close liaison with other in-country Movement partners: International Committee of the Red Cross (ICRC), German Red Cross (GRC), Norwegian Red Cross (NorCross) and Turkish Red Crescent Society (TRCS) – who are likely to support the National Society’s response. Other partner organizations actively involved in the operation: National Disaster Management Authority (NDMA), Provincial Disaster Management Authorities (PDMAs), District Administration, United Nations (UN) and local NGOs. Summary of major revisions made to emergency plan of action: Another round of continuous heavy rains started in most part of the country on the week of 20 August 2020 until 3 September 2020 intermittently. The second round of torrential rains caused urban flooding in the Sindh province and flash flooding in Khyber Pakhtunkhwa (KP). New areas have been affected by the urban flooding including the districts of Malir, Karachi Central, Karachi West, Karachi East and Korangi (Sindh), and District Shangla, Swat and Charsadda in Khyber Pakhtunkhwa. -

Sindh Coast: a Marvel of Nature
Disclaimer: This ‘Sindh Coast: A marvel of nature – An Ecotourism Guidebook’ was made possible with support from the American people delivered through the United States Agency for International Development (USAID). The contents are the responsibility of IUCN Pakistan and do not necessarily reflect the opinion of USAID or the U.S. Government. Published by IUCN Pakistan Copyright © 2017 International Union for Conservation of Nature. Citation is encouraged. Reproduction and/or translation of this publication for educational or other non-commercial purposes is authorised without prior written permission from IUCN Pakistan, provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission from IUCN Pakistan. Author Nadir Ali Shah Co-Author and Technical Review Naveed Ali Soomro Review and Editing Ruxshin Dinshaw, IUCN Pakistan Danish Rashdi, IUCN Pakistan Photographs IUCN, Zahoor Salmi Naveed Ali Soomro, IUCN Pakistan Designe Azhar Saeed, IUCN Pakistan Printed VM Printer (Pvt.) Ltd. Table of Contents Chapter-1: Overview of Ecotourism and Chapter-4: Ecotourism at Cape Monze ....... 18 Sindh Coast .................................................... 02 4.1 Overview of Cape Monze ........................ 18 1.1 Understanding ecotourism...................... 02 4.2 Accessibility and key ecotourism 1.2 Key principles of ecotourism................... 03 destinations ............................................. 18 1.3 Main concepts in ecotourism ................. -

The Geographic, Geological and Oceanographic Setting of the Indus River
16 The Geographic, Geological and Oceanographic Setting of the Indus River Asif Inam1, Peter D. Clift2, Liviu Giosan3, Ali Rashid Tabrez1, Muhammad Tahir4, Muhammad Moazam Rabbani1 and Muhammad Danish1 1National Institute of Oceanography, ST. 47 Clifton Block 1, Karachi, Pakistan 2School of Geosciences, University of Aberdeen, Aberdeen AB24 3UE, UK 3Geology and Geophysics, Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA 4Fugro Geodetic Limited, 28-B, KDA Scheme #1, Karachi 75350, Pakistan 16.1 INTRODUCTION glaciers (Tarar, 1982). The Indus, Jhelum and Chenab Rivers are the major sources of water for the Indus Basin The 3000 km long Indus is one of the world’s larger rivers Irrigation System (IBIS). that has exerted a long lasting fascination on scholars Seasonal and annual river fl ows both are highly variable since Alexander the Great’s expedition in the region in (Ahmad, 1993; Asianics, 2000). Annual peak fl ow occurs 325 BC. The discovery of an early advanced civilization between June and late September, during the southwest in the Indus Valley (Meadows and Meadows, 1999 and monsoon. The high fl ows of the summer monsoon are references therein) further increased this interest in the augmented by snowmelt in the north that also conveys a history of the river. Its source lies in Tibet, close to sacred large volume of sediment from the mountains. Mount Kailas and part of its upper course runs through The 970 000 km2 drainage basin of the Indus ranks the India, but its channel and drainage basin are mostly in twelfth largest in the world. Its 30 000 km2 delta ranks Pakiistan. -
Sialkot Blockwise
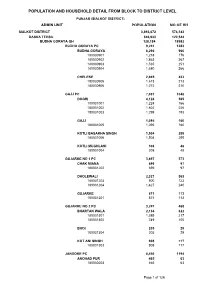
POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (SIALKOT DISTRICT) ADMIN UNIT POPULATION NO OF HH SIALKOT DISTRICT 3,893,672 574,143 DASKA TEHSIL 846,933 122,544 BUDHA GORAYA QH 128,184 18982 BUDHA GORAYA PC 9,241 1383 BUDHA GORAYA 6,296 960 180030901 1,218 176 180030902 1,863 267 180030903 1,535 251 180030904 1,680 266 CHELEKE 2,945 423 180030905 1,673 213 180030906 1,272 210 GAJJ PC 7,031 1048 DOGRI 4,124 585 180031001 1,224 166 180031002 1,602 226 180031003 1,298 193 GAJJ 1,095 160 180031005 1,095 160 KOTLI BASAKHA SINGH 1,504 255 180031006 1,504 255 KOTLI MUGHLANI 308 48 180031004 308 48 GUJARKE NO 1 PC 3,897 573 CHAK MIANA 699 97 180031202 699 97 DHOLEWALI 2,327 363 180031203 900 123 180031204 1,427 240 GUJARKE 871 113 180031201 871 113 GUJARKE NO 2 PC 3,247 468 BHARTAN WALA 2,134 322 180031301 1,385 217 180031302 749 105 BHOI 205 29 180031304 205 29 KOT ANI SINGH 908 117 180031303 908 117 JANDOKE PC 8,450 1194 ANOHAD PUR 465 63 180030203 465 63 Page 1 of 126 POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (SIALKOT DISTRICT) ADMIN UNIT POPULATION NO OF HH JANDO KE 2,781 420 180030206 1,565 247 180030207 1,216 173 KOTLI DASO SINGH 918 127 180030204 918 127 MAHLE KE 1,922 229 180030205 1,922 229 SAKHO KE 2,364 355 180030201 1,250 184 180030202 1,114 171 KANWANLIT PC 16,644 2544 DHEDO WALI 6,974 1092 180030305 2,161 296 180030306 1,302 220 180030307 1,717 264 180030308 1,794 312 KANWAN LIT 5,856 854 180030301 2,011 290 180030302 1,128 156 180030303 1,393 207 180030304 1,324 201 KOTLI CHAMB WALI -

Institution Wise Pass Percentage SSC 9Th Annual Examination 2017
BOARD OF INTERMEDIATE & SECONDARY EDUCATION, MULTAN A-7 1 INSTITUTION WISE PASS PERCENTAGE AND GRADING 9TH EXAM 2017 Appeared Passed Pass% Appeared Passed Pass% 101001 Govt. Girls English Medium Model 101026 Govt. Girls High School 80/10-R High School, Khanewal Pirowal, Khanewal 787 514 65.31 103 63 61.17 101002 Govt. M.C .E/M Girls High School, 101028 GOVT. GIRLS HIGH SCHOOL BAGAR Khanewal SARGANA, Kabirwala 181 131 72.38 30 27 90.00 101003 Govt. Girls High School, 101029 Govt. Girls High School Nanakpur Kabirwala ,Khanewal 473 207 43.76 44 23 52.27 101004 Govt. Girls Model High School, 101030 Govt. Girls Hassan Model High Mian Channu School, Khanewal. 578 411 71.11 280 179 63.93 101005 Govt. Girls E/M High School, 101031 Govt. Girls High School 92/10.R, Jahanian Khanewal 425 229 53.88 34 25 73.53 101008 Govt. Methodist Girls High School 101032 Govt. Girls High School, 28/10-R 135/16-L, Khanewal ,Khanewal 118 42 35.59 36 33 91.67 101010 Govt. Girls High School 136/10-R, 101033 Govt. Girls High School 23/10-R, Khanewal Khanewal 106 64 60.38 70 40 57.14 101011 Govt. Girls E/M High School, 101034 Govt. Girls High School, 72/10-R 138/10-R, Jahanian ,Khanewal 119 104 87.39 45 13 28.89 101012 Govt. Girls High School, 12/A.H. 101035 Govt. Girls High School, 116/15-L ,Khanewal Mainchannu ,Khanewal 111 77 69.37 33 22 66.67 101013 Govt. Girls High School Sarai 101036 Govt.