Running Title: Reproduction of Primula Bergidensis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Mencan Rock Garden Society
Bulletin of the mencan Rock Garden Society VOL. 42 50th Anniversary Issue NO. 5 THE BULLETIN Editor Laura Louise Foster, Falls Village, Conn. 06031 Assistant Editor Harry Dewey, 4605 Brandon Lane, Beltsville, MD. 20705 Contributing Editors Roy Davidson, Anita Kistler, H. Lincoln Foster, Owen Pearce, H.N. Porter Layout Designer Buffy Parker Advertising Manager . .Anita Kistler, 1421 Ship Rd., West Chester, Pa. 19380 ANNIVERSARY ISSUE CONTENTS VOL. 42 NO. 5 1984 The Anniversary Celebration —L.L. Foster 1 The Pre-Conference Tour—Judy Glattstein 12 The Post-Conference Tour—Nickolas Nickou 18 As It Was in the Beginning—F.H. Cabot 22 The ARGS Hymn 51 Illustrations—Laura Louise Foster Published quarterly by the AMERICAN ROCK GARDEN SOCIETY, a tax-exempt, non-profit organization incorporated under the laws of the state of New Jersey. You are invited to join. Annual dues (Bulletin included), to be submitted in U.S. Funds or International Money Order, are: General Membership, $15.00 (includes domestic or foreign, single or joint—2 at same address to receive 1 Bulletin, 1 Seed List); Patron, $50.00; Life Member, $250.00. Membership inquiries and dues should be sent to Norman Singer, Secretary, SR 66 Box 114, Norfolk Rd., Sandisfield, Mass. 01255. The office of publication is located at Norfolk Rd., Sandisfield, Mass. 01255. Address editorial matters per• taining to the Bulletin to the Editor, Laura Louise Foster. Falls Village, Conn. 06031. Address advertising matters to Anita Kistler, 1421 Ship Rd., West Chester, Pa. 19380. Second Class Postage paid in Sandisfield, Mass. and additional offices. Bulletin of the American Rock Garden Society (ISSN 0003-0864). -

(Dr. Sc. Nat.) Vorgelegt Der Mathematisch-Naturwissenschaftl
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2012 Flowers, sex, and diversity: Reproductive-ecological and macro-evolutionary aspects of floral variation in the Primrose family, Primulaceae de Vos, Jurriaan Michiel Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-88785 Dissertation Originally published at: de Vos, Jurriaan Michiel. Flowers, sex, and diversity: Reproductive-ecological and macro-evolutionary aspects of floral variation in the Primrose family, Primulaceae. 2012, University of Zurich, Facultyof Science. FLOWERS, SEX, AND DIVERSITY. REPRODUCTIVE-ECOLOGICAL AND MACRO-EVOLUTIONARY ASPECTS OF FLORAL VARIATION IN THE PRIMROSE FAMILY, PRIMULACEAE Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr. sc. nat.) vorgelegt der Mathematisch-naturwissenschaftliche Fakultät der Universität Zürich von Jurriaan Michiel de Vos aus den Niederlanden Promotionskomitee Prof. Dr. Elena Conti (Vorsitz) Prof. Dr. Antony B. Wilson Dr. Colin E. Hughes Zürich, 2013 !!"#$"#%! "#$%&$%'! (! )*'+,,&$-+''*$.! /! '0$#1'2'! 3! "4+1%&5!26!!"#"$%&'(#)$*+,-)(*#! 77! "4+1%&5!226!-*#)$%.)(#!'&*#!/'%#+'.0*$)/)"$1'(12%-).'*3'0")"$*.)4&4'*#' "5*&,)(*#%$4'+(5"$.(3(-%)(*#'$%)".'(#'+%$6(#7.'2$(1$*.".! 89! "4+1%&5!2226!.1%&&'%#+',!&48'%'9,%#)()%)(5":'-*12%$%)(5"'"5%&,%)(*#'*3' )0"';."&3(#!'.4#+$*1"<'(#'0")"$*.)4&*,.'%#+'0*1*.)4&*,.'2$(1$*.".! 93! "4+1%&5!2:6!$"2$*+,-)(5"'(12&(-%)(*#.'*3'0"$=*!%14'(#'0*1*.)4&*,.' 2$(1$*.".>'5%$(%)(*#'+,$(#!'%#)0".(.'%#+'$"2$*+,-)(5"'%..,$%#-"'(#' %&2(#"'"#5($*#1"#).! 7;7! "4+1%&5!:6!204&*!"#")(-'%#%&4.(.'*3'!"#$%&''."-)(*#'!"#$%&''$"5"%&.' $%12%#)'#*#/1*#*204&4'%1*#!'1*2$0*&*!(-%&&4'+(.)(#-)'.2"-(".! 773! "4+1%&5!:26!-*#-&,+(#!'$"1%$=.! 7<(! +"=$#>?&@.&,&$%'! 7<9! "*552"*?*,!:2%+&! 7<3! !!"#$$%&'#""!&(! Es ist ein zentrales Ziel in der Evolutionsbiologie, die Muster der Vielfalt und die Prozesse, die sie erzeugen, zu verstehen. -

This Article Appeared in a Journal Published by Elsevier. the Attached Copy Is Furnished to the Author for Internal Non-Commerci
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Review of Palaeobotany and Palynology 160 (2010) 163–171 Contents lists available at ScienceDirect Review of Palaeobotany and Palynology journal homepage: www.elsevier.com/locate/revpalbo Pollen morphology, ultrastructure and taphonomy of the Neuradaceae with special reference to Neurada procumbens L. and Grielum humifusum E.Mey. ex Harv. et Sond. S. Polevova a, M. Tekleva b,⁎, F.H. Neumann c,d, L. Scott e, J.C. Stager f a Moscow State University, Moscow, Russia b Borissyak Paleontological Institute RAS, Moscow, Russia c Bernard Price Institute for Palaeontology, University of the Witwatersrand, Johannesburg, South Africa d Steinmann Institute for Geology, Mineralogy and Palaeontology, University of Bonn, Germany e Department of Plant Sciences, University of the Free State, Bloemfontein, South Africa f Paul Smiths College, New York, USA article info abstract Article history: Pollen morphology and sporoderm ultrastructure of modern Neurada procumbens L. and Grielum humifusum Received 5 November 2009 E.Mey. ex Harv. -

Vascular Flora and Geoecology of Mont De La Table, Gaspésie, Québec
RHODORA, Vol. 117, No. 969, pp. 1–40, 2015 E Copyright 2015 by the New England Botanical Club doi: 10.3119/14-07; first published on-line March 11, 2015. VASCULAR FLORA AND GEOECOLOGY OF MONT DE LA TABLE, GASPE´ SIE, QUE´ BEC SCOTT W. BAILEY USDA Forest Service, 234 Mirror Lake Road, North Woodstock, NH 03262 e-mail: [email protected] JOANN HOY 21 Steam Mill Road, Auburn, NH 03032 CHARLES V. COGBILL 82 Walker Lane, Plainfield, VT 05667 ABSTRACT. The influence of substrate lithology on the distribution of many vascular and nonvascular plants has long been recognized, especially in alpine, subalpine, and other rocky habitats. In particular, plants have been classified as dependent on high-calcium substrates (i.e., calcicoles) based on common restriction to habitats developed in calcareous rocks, such as limestone and marble. In a classic 1907 paper on the influence of substrate on plants, M. L. Fernald singled out a particular meadow on Mont de la Table in the Chic-Choc Mountains of Que´bec for its unusual co-occurrence of strict calcicole and calcifuge (i.e., acidophile) plant taxa. We re-located this site, investigated substrate factors responsible for its unusual plant diversity, and documented current plant distributions. No calcareous rocks were found on site. However, inclusions of calcareous rocks were found farther up the mountain. The highest pH and dissolved calcium concentrations in surface waters were found in a series of springs that deliver groundwater, presumably influenced by calcareous rocks up the slope. Within the habitat delineated by common occurrences of calcicole species, available soil calcium varied by a factor of five and soil pH varied by almost 1.5 units, depending on microtopography and relative connection with groundwater. -

Dissertation
DISSERTATION Titel der Dissertation Exudate flavonoids in Primulaceae: comparative studies of chemodiversity aspects Verfasserin Mag. rer. nat. Tshering Doma Bhutia angestrebter akademischer Grad Doktorin der Naturwissenschaften (Dr. rer. nat.) Wien, 2013 Studienkennzahl lt. Studienblatt: A 091 438 Dissertationsgebiet lt. Studienblatt: Botanik Betreuerin / Betreuer: Ao. Univ. Prof. Dr. Karin Valant-Vetschera Acknowledgements It is my great pleasure to thank all those who, with their help and support, have contributed to the completion of this thesis. First and foremost I would like to express my sincere and heartfelt gratitute to my supervisor Assoc. Prof. Dr. Karin Valant‐Vetschera for giving me the opportunity to join the “Chemodiversity Group”. I thank her for assuming the dual role of supervisor and mentor. During the years of my diploma and doctoral theses she has continuously offered me the best guidance, support and advice I could have asked for. I am very grateful to Dr. Lothar Brecker for the characterization and identification of the isolated compounds. Additionally, I thank him for his constant encouragement, support and valuable suggestions. In the lab I have always received invaluable technical support from Mag. Johann Schinnerl, for which I extend him my earnest thanks and appreciation. Prof. Dr. Harald Greger has been very kind and supportive throughout the years, which I gratefully appreciate. Thanks are also due to Prof. Dr. Irene Lichtscheidl and Dr. Wolfram Adlassnig for providing access to their laboratory equipment and for their excellent guidance. I am deeply indebted to Prof. Eckhard Wollenweber (Institut für Botanik der TU Darmstadt, Germany) for the constant supply of authentic flavonoid samples, which made my lab life a lot easier. -
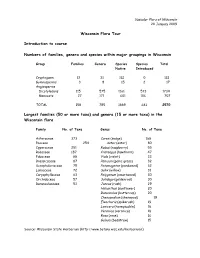
Wisconsin Flora Tour Introduction to Course Numbers of Families, Genera
Vascular Flora of Wisconsin 20 January 2009 Wisconsin Flora Tour Introduction to course Numbers of families, genera and species within major groupings in Wisconsin Group Families Genera Species Species Total Native Introduced Cryptogams 13 31 112 0 112 Gymnosperms 3 8 15 2 17 Angiosperms Dicotyledons 115 575 1161 573 1734 Monocots 27 171 601 106 707 TOTAL 158 785 1889 681 2570 Largest families (50 or more taxa) and genera (15 or more taxa) in the Wisconsin flora Family No. of Taxa Genus No. of Taxa Asteraceae 373 Carex (sedge) 168 Poaceae 254 Aster (aster) 80 Cyperaceae 251 Rubus (raspberry) 55 Rosaceae 187 Crateagus (hawthorn) 47 Fabaceae 88 Viola (violet) 33 Brassicaceae 87 Panicum (panic grass) 32 Scrophulariaceae 75 Potamogeton (pondweed) 32 Lamiaceae 72 Salix (willow) 31 Caryophyllaceae 63 Polygonum (smartweed) 30 Orchidaceae 57 Solidago (goldenrod) 30 Ranunculaceaee 53 Juncus (rush) 29 Helianthus (sunflower) 20 Ranunculus (buttercup) 20 Chenopodium (chenopod) 19 Eleocharis (spikerush) 19 Lonicera (honeysuckle) 18 Veronica (veronica) 18 Rosa (rose) 16 Galium (bedstraw) 15 Source: Wisconsin State Herbarium (http://www.botany.wisc.edu/herbarium/) Four major floristic elements in the Wisconsin flora Boreal Alleghenian Ozarkian Prairie Two floristic provinces Northern hardwood Prairie forests Tension Zone Brief look at four plant communities Beech maple or southern mesic Oak forest or southern xeric Prairie Bog or fen Vascular Flora of Wisconsin 22 January 2009 Nomenclature and Vascular Cryptogams I Nomenclature vs. Classification Rank -

Reproductive Biology of Island and Mainland Populations of Primula Mistassinica (Primulaceae) on Lake Huron Shorelines
1819 Reproductive biology of island and mainland populations of Primula mistassinica (Primulaceae) on Lake Huron shorelines Brendon M.H. Larson and Spencer C.H. Barrett Abstract: To investigate the influence of insularity on plant reproductive biology at a local geographic scale, we examined aspects of reproduction in distylous Primula mistassinica Michx. (Primulaceae) on Lake Huron shorelines of the Bruce Peninsula and adjacent Tobermory Islands in Ontario, Canada. A total of 7 mainland and 13 nearshore island populations were compared. Controlled pollinations demonstrated that P. mistassinica possesses a dimorphic incompatibility system with intermorph crosses setting significantly more seeds than self or intramorph crosses. Floral morphology, population style-morph ratios, and seed fertility were compared in mainland and nearshore island populations to determine whether there was evidence for differences in reproductive traits between these areas. Style-morph ratios did not differ significantly from equilibrium expectations, and there were no consistent differences between island and mainland populations in floral morphology or fertility. Rather, the generalized pollination system of P. mistassinica and extensive historical opportunities for colonization appear to have mitigated insular effects so that proximate ecological factors are more relevant to the current reproductive biology of populations. Key words: distyly, insularity, pollination, reproductive biology. Résumé : Afin d’étudier l’influence de l’insularité sur la biologie reproductive des plantes à l’échelle géographique locale, les auteurs ont examiné des aspects de la reproduction chez le Primula mistassinica Michx. (Primulaceae) distyle, venant sur les rives du lac Huron bordant la péninsule de Bruce et sur les îles Tobermory adjacentes, en Ontario, au Canada. Ils ont comparé au total 7 populations continentales et 13 populations insulaires riveraines. -

Conservation of Primula Farinosa in Poland with Respect to the Genetic
Ǘ Ǘ Acta Societatis Botanicorum Poloniae DOI: 10.5586/asbp.3577 ORIGINAL RESEARCH PAPER Publication history Received: 2017-09-25 Accepted: 2018-04-06 Conservation of Primula farinosa in Poland Published: 2018-06-04 with respect to the genetic structure of Handling editor Zygmunt Dajdok, Faculty of populations Biological Sciences, University of Wrocław, Poland Authors’ contributions Zbigniew Gajewski 1, Piotr Boroń 2, Anna Lenart-Boroń 3, Barbara ZG: research design, writing 1 1 4 the manuscript, collecting Nowak *, Ewa Sitek , Józef Mitka plant material, and preparing 1 Department of Botany and Plant Physiology, Faculty of Biotechnology and Horticulture, samples; PB: conducting University of Agriculture in Krakow, al. 29 Listopada 54, 31-425 Krakow, Poland analysis, writing the manuscript; 2 Department of Forest Pathology, Mycology and Tree Physiology, Faculty of Forestry, University ALB: conducting analysis, of Agriculture in Krakow, al. 29 Listopada 46, 31-425 Krakow, Poland writing the manuscript; 3 Department of Microbiology, Faculty of Agriculture and Economics, University of Agriculture in BN: writing the manuscript, Krakow, al. Mickiewicza 24/28, 30-059 Krakow, Poland collecting plant material; ES: 4 Botanic Garden of the Jagiellonian University, Kopernika 27, 31-501 Krakow, Poland research design, collecting plant material; JM: comments * Corresponding author. Email: [email protected] on results Funding Abstract The research was conducted within the framework of the e bird’s-eye primrose ( Primula farinosa L.) is an endangered species in Poland. “Active and conservative e sole remaining, and critically endangered, population of approximately 300 protection of bird’s-eye flowering plants is located in the Beskid Sądecki Mountains (Jaworki, Poland). -

Serotiny in Primula Palinuri: How to Face the Dry Season on Mediterranean Cliffs
diversity Article Serotiny in Primula palinuri: How to Face the Dry Season on Mediterranean Cliffs Roberto Silvestro 1,2, Luigi Gennaro Izzo 1,* , Maurizio Buonanno 3 and Giovanna Aronne 1 1 Department of Agricultural Sciences, University of Naples Federico II, Via Università 100, 80055 Portici, Italy; [email protected] (R.S.); [email protected] (G.A.) 2 Département des Sciences Fondamentales, Université du Québec à Chicoutimi, 555 boulevard de l’Université, Chicoutimi, QC G7H2B1, Canada 3 Institute for Mediterranean Agricultural and Forestry Systems (ISAFoM), National Research Council (CNR), P.le Enrico Fermi 1-Loc. Porto del Granatello, 80055 Portici, Italy; [email protected] * Correspondence: [email protected] Received: 9 June 2020; Accepted: 22 July 2020; Published: 25 July 2020 Abstract: Primula palinuri Petagna is the only Mediterranean and maritime species in the genus Primula, is endemic to coastal cliffs of southern Italy, and is classified as endangered with a decreasing population trend in the IUCN Red List. For this species, the major bottleneck for long-term survival has been recognized to be recruitment failure. In this study, we investigated the seed release strategy of P. palinuri, by using field observations and laboratory experiments. We hypothesized that repetitive cycles of wet/dry conditions and external wax removal could be the environmental triggers of capsule dehiscence. Data showed that capsules treated with wet/dry cycles dehisced within 75 days, while none subjected to constant dry conditions dehisced. Once dehisced, capsules repetitively closed when made wet, and opened again upon drying. Seeds of P. palinuri can remain on plant up to 2 years, over which time capsules reclose when rained upon and reopen upon drying, highlighting the first reported occurrence of serotiny in a Primula species. -

Characterization of Novel Microsatellite Loci for Primula Poissonii (Primulaceae) Using High-Throughput Sequencing Technology
molecules Article Characterization of Novel Microsatellite Loci for Primula poissonii (Primulaceae) Using High-Throughput Sequencing Technology Yun-Jiao Liu 1,2, Cai-Yun Zhang 2, Gang Hao 2, Xue-Jun Ge 1 and Hai-Fei Yan 1,* 1 Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China; [email protected] (Y.-J.L.); [email protected] (X.-J.G.) 2 College of Life Sciences, South China Agricultural University, Guangzhou 510642, China; [email protected] (C.-Y.Z.); [email protected] (G.H.) * Correspondence: [email protected]; Tel.: +86-20-3708-5659; Fax: +86-20-3725-2551 Academic Editor: Derek J. McPhee Received: 24 January 2016; Accepted: 19 April 2016; Published: 9 May 2016 Abstract: Primula poissonii (Primulaceae) is a perennial herb, widely distributed in the Hengduan Mountain region of Southwest China. In this study, Roche 454 pyrosequencing was used to isolate microsatellite markers. A total of 4528 unique sequences were identified from 68,070 unique reads. Of these, eighty-seven microsatellite loci were screened for utility using two criteria: successful PCR amplification and variation of these loci within three wild P. poissonii populations. Twenty loci were successfully amplified and exhibited polymorphic alleles. The number of observed alleles ranged from 1 to 9 with an average of 3.5. The observed and expected heterozygosities ranged from 0.087 to 1.000 and from 0.124 to 0.828, respectively. Among these SSR loci, only the P69 locus could not be cross-amplified successfully in two closely related species P. -

Carex Atratiformis Britton Sedge
sedge, Page 1 Carex atratiformis Britton sedge State Distribution Photo by Susan R. Crispin Best Survey Period Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Status: State threatened Ontario, and Vermont, and is known only from historical records in New Hampshire (NatureServe 2007). Global and state rank: G5/S2 State distribution: This rare sedge is known from 13 Other common names: black sedge sites, with all but two occurring in Isle Royale National Park. The two mainland localities, both of which are Family: Cyperaceae (sedge family) historical records, are comprised of an 1863 collection in Keweenaw County, and a 1914 collection in Marquette Synonyms: Carex ovata Rudge; C. atrata L. subsp. County. Within the Isle Royale archipelago, C. atratiformis (Britton) Kükenthal; C. atratiformis subsp. atratiformis occurs from the main island to Passage raymondii (Calder) A.E. Porsild; C. raymondii Calder Island, where it is often frequent over relatively large (Flora of North America 2002). areas, including the coasts of several near-shore, small island chains and along significant portions of several of Taxonomy: This taxon includes a minor variant that the many protected bays, coves, and harbors of the main was formerly referred to C. raymondii based on small island. pigmentation differences in the perigynia and scales; C. atratiformis is also known to hybridize with the related Recognition: C. atratiformis is a loosely tufted sedge C. norvegica Retzius where their ranges overlap in ranging up to ca. 70 dm in height, with strongly reddish Newfoundland and Quebec, forming the hybrid C. stem bases and clumps of 2-5 mm-wide leaves that tend ×quirponensis Fernald (Flora of North America 2002).