Arene Life Sciences Limited, Unit‐I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redgram Outlook February 2021
Redgram Outlook, February 2021 Redgram Outlook – February 2021 Redgram is commonly known as Tur or Arhar in India and is the second important pulse crop in the country after gram (chana). The ability of redgram to produce high economic yields under soil moisture deficit makes it an important crop in rainfed and dry land agriculture. World major redgram producing countries are India (37.50 lakh tonnes), Myanmar (6.76 lakh tonnes), Malawi (4.34 lakh tonnes), Tanzania (3.15 lakh tonnes) and Haiti (0.87 lakh tonnes). Area under redgram reported during 2020-21 was 48.24 lakh ha (119.20 lakh acres) as against 45.45 lakh ha (112.31 lakh acres) during the same period in 2019-20. In India, major redgram producing states are Karnataka 12.80 lakh ha (31.63 lakh acres), Maharashtra 12.46 lakh ha (30.79 lakh acres), Telangana 4.34 lakh ha (10.72 lakh acres), Madhya Pradesh 4.12 lakh ha (10.18 lakh acres) and Uttar Pradesh 3.53 lakh ha (8.72 lakh acres). According to Government 1st advance estimates, all India redgram production in 2020-21 is at 4.04 million tonnes. 4.87 4.29 4.04 3.83 3.32 3.02 3.17 2.81 2.65 2.56 2011-12 2012-13 2013-14 2014-15 2015-16 2016-17 2017-18 2018-19 2019-20 2020-21* Source: Directorate of Economics and Statistics (DES). Figure 1: Production of Redgram in India (in million tonnes) Agricultural Market Intelligence Centre, PJTSAU Page 1 Redgram Outlook, February 2021 Table 1: Redgram Domestic Supply & Demand (in lakh tonnes) 2018-19 2019-20 2020-21* Opening Stocks 8.49 6.66 7.71 Production 33.20 38.30 40.4 Imports 5.30 5.00 5 Total Supply 46.99 49.96 53.11 Exports 0.33 0.25 0.4 Consumption 40.00 42.00 43.5 Total Demand 40.33 42.25 43.9 Ending Stocks 6.66 7.71 9.21 Source: www.agriwatch.com *Estimated based on 1st Advance Estimates of Government of India The major markets for this crop in Telangana are Badepalli, Devarakadra, Gadwal, Mahabubnagar, Narayanpet, Sadasivpet, Zaheerabad, Suryapet, Tandur and Warangal. -

Meos & MIS Co-Ordinators
List of MEOs, MIS Co-orfinators of MRC Centers in AP Sl no District Mandal Name Designation Mobile No Email ID Remarks 1 2 3 4 5 6 7 8 1 Adilabad Adilabad Jayasheela MEO 7382621422 [email protected] 2 Adilabad Adilabad D.Manjula MIS Co-Ordinator 9492609240 [email protected] 3 Adilabad ASIFABAD V.Laxmaiah MEO 9440992903 [email protected] 4 Adilabad ASIFABAD G.Santosh Kumar MIS Co-Ordinator 9866400525 [email protected] [email protected] 5 Adilabad Bazarhathnoor M.Prahlad MEO(FAC) 9440010906 n 6 Adilabad Bazarhathnoor C.Sharath MISCo-Ord 9640283334 7 Adilabad BEJJUR D.SOMIAH MEO FAC 9440036215 [email protected] MIS CO- 8 Adilabad BEJJUR CH.SUMALATHA 9440718097 [email protected] ORDINATOR 9 Adilabad Bellampally D.Sridhar Swamy M.E.O 7386461279 [email protected] 10 Adilabad Bellampally L.Srinivas MIS CO Ordinator 9441426311 [email protected] 11 Adilabad Bhainsa J.Dayanand MEO 7382621360 [email protected] 12 Adilabad Bhainsa Hari Prasad.Agolam MIS Co-ordinator 9703648880 [email protected] 13 Adilabad Bheemini K.Ganga Singh M.E.O 9440038948 [email protected] 14 Adilabad Bheemini P.Sridar M.I.S 9949294049 [email protected] 15 Adilabad Boath A.Bhumareedy M.E.O 9493340234 [email protected] 16 Adilabad Boath M.Prasad MIS CO Ordinator 7382305575 17 Adilabad CHENNUR C.MALLA REDDY MEO 7382621363 [email protected] MIS- 18 Adilabad CHENNUR CH.LAVANYA 9652666194 [email protected] COORDINATOR 19 Adilabad Dahegoan Venkata Swamy MEO 7382621364 [email protected] 20 -

SANGAREDDY ASSEMBLY CONSTITUENCY Sl.No
F O R M 7 A ( See Rule 10 ( 1) LIST OF CONTESTING CANDIDATES ELECTION TO THE A.P.L.A. 2009 39- SANGAREDDY ASSEMBLY CONSTITUENCY Sl.No. Name of Candidate Address of Candidate Party affiliation Symbol Allotted 1 2 3 4 5 1 GADILA NAWAZ REDDY 3-5-48, Ward 3 Block NO 5,6 & 9 , TELANGANA RASTRA CAR Sadasivpet town & Mandal SAMITHI 2 N. CHANDRA SHEKAR 2-7-22, Ram Nagar Sangareddy BHARATIYA JANATH PARTY LOTUS Town & Mandal 3 CHINTA PRABHAKER 4-1-1/8/5 Sadasiva Enclave TELUGU DESHAM BYCLE Sadasivpet town & Mandal 4 JAYA PRAKASH REDDY .T 2-6-131 Ram Nagar Sangareddy INDIAN NATIONAL HAND town & Mandal CONGRESS 5 T. NARSIMULU 4-24, Marepally (V) Kondapur BHAHUJAN SAMAJ PARTY ELEPHANT Mandal 6 CHALAMALA YASODA 9-9 Bank Colony Pothireddipally (v) PYRAMID PARTY OF INDIA TELEVISION LAKSHMI Sangareddy Mandal 7 PATLOLLA MADHAVA 1-37 Togarpally (v) Kondapur LOK SATTA PARTY WHISTLE REDDY Mandal 8 FAHEEM .M.A. 1-3, Ilapur (V) Patancheru (M) PRAJA RAJYAM PARTY RAILWAY ENGINE 9 SADAKULA KRISHNAIAH 2-77, Malkapur (V) Kondapur TRILINGA PRAJA PRAGATHI CEILING FAN Mandal PARTY 10 ANKENA PALLI 1-1-95, Ram Mandir Road , INDEPENDENT SHUTTLE SHRISHAILAM Sadasivpet town & Mandal 11 AZHAR MOHD. 5-1-11/4, Shanthi Nagar INDEPENDENT BALOON Sangareddy town & Mandal 12 ATAUR RAHMAN 9-8-109/A/3, Chota Bazar , INDEPENDENT BANANA Golkonda, Kharwan, Hyderabad 13 ANANTHA RAO KULAKARNI H.No. 4-7-8/1/B2 Revenue Colony INDEPENDENT CO-CONUT Sangareddy town & Mandal 14 GADDAMADA NARSIMULU 3-90/1, Indrakaran (V), Sangareddy INDEPENDENT KITE (M) 15 TAMMALI NARSIMULU 1-5-49, Bramman wada INDEPENDENT SLATE Sangareddy town & Mandal 16 TAHERA 1-7, Suraram (V) Sadasivpet INDEPENDENT RING Mandal 17 P. -

Details of Staff Working at Dist. / Constituency / Mandal Level As on 27-07-2019
GOVERNMENT OF TELANGANA DEPARTMENT OF HORTICULTURE & SERICULTURE Details of Staff Working at Dist. / Constituency / Mandal Level as on 27-07-2019 INDEX Page Numbers Page Numbers S.No District Name S.No District Name From -- To From -- To 1 Adilabad 1 to 2 17 Mahabubnagar 29 to 30 2 Nirmal 3 to 4 18 Narayanapet 31 3 Mancherial 5 to 6 19 Nagarkurnool 32 to 33 4 Komarambheem 7 20 Gadwal 34 to 35 5 Karimnagar 8 to 9 21 Wanaparthy 36 to 37 6 Peddapalli 10 22 Vikarabad 38 to 39 7 Jagityal 11 to 12 23 Rangareddy 40 to 41 8 Siricilla 13 24 Medchal 42 9 Warangal ( R) 14 to 15 25 Sangareddy 43 to 44 10 Warangal(U) 16 to 17 26 Medak 45 to 46 11 Bhupalapally 18 to 19 27 Siddipiet 47 to 48 12 Mulugu 20 28 Nizamabad 49 to 50 13 Mahbubabad 21 to 22 29 Kamareddy 51 to 52 14 Jangaon 23 to 24 30 Nalgonda 53 to 55 15 Khammam 25 to 26 31 Suryapet 56 to 57 16 Kothagudem 27 to 28 32 Yadadri 58 to 59 Statement showing the Officer & Staff working in Horticulture & Sericulture Department No. of Assembly Constituencies : 2 Adilabad Constituency = 5 mandals Boath Constituency = 9 mandals Name of the new District:- No. of Mandals : 18 ADILABAD Part of Khanapur Constituency = 2 mandals Part of Asifabad constituency = 2 mandals Total mandals = 18 Sl. Name of the Employee Head Quarters / Assembly No. of Name of the Designation Name of the Mandals (Jurisdiction) No. Sarvasri/ Smt./ Kum. Constituency Mandals MLH&SO A - District Level Horticulture & Sericulture Officer Mulug, Venkatapur, Govindaraopet, K.Venkateshwarlu PD/ DH&SO, Adilabad 1 Adilabad Tadvai, Eturnagaram, Mangapet, 7997724995 (DDO - Adilabad ) Kannaigudem, Wajedu, Venkatapuram B - Constituency Level Officers Adilabad 1 Adilabad (R), Ch.Pranay Reddy MLH&SO(MIP) G.Srinivas Boath 1 Bheempur 7997725008 1 HO(T)/ CLH&SO 7997725002 A. -

Draft Electoral Roll of Medak-Nizamabad-Adilabad-Karimnagar Teachers Constituency of the A.P Legislative Council As Published on 15-12-2012
Draft Electoral Roll of Medak-Nizamabad-Adilabad-Karimnagar Teachers Constituency of the A.P Legislative Council as published on 15-12-2012 Polling Station Number : ( 95 ) Kangti District: Medak - 17 MPCPS Kangti Sl.No. House address Full Name of the Name of father/ mother / Name of educational Age (Place of ordinary elector husband institution, if any, in residence) which he is teaching (1) (2) (3) (4) (5) (6) Mandal : KANGTI Village: BHANSWADA 1-37/2 Maheshwar Rao Karanam Bhim Rao ZPHS , Siragapur 41 1 Banswada Mandal : KANGTI Village: CHAPTA [KHURD] 1-13 Santoosh Narayana ZPHS Tadkal 31 2 chapta (K) Mandal : KANGTI Village: CHIMAL PAHAD 22 Shamappa Mallappa ZPHS akgnti 28 3 Chemalphad Mandal : KANGTI Village: DEGULAWADI 3-56 Nulen Sanjeev Sri.Ramappa GHS Shankarampet A 28 4 Degulwadi Mandal : KANGTI Village: GAJULPAHAD 2-54 Jadhave Ganapathi Shankar ZPHS, Bibipet (V) kalher (M) 27 5 Gajulpad Mandal : KANGTI Village: JAMGIBURG 2-5-C/1 Kesari Anjaiah Kesari Ramaiah ZPHS Hanmanthrao pet 35 6 Gandhia nagar Gandhi nagar Mandal : KANGTI Village: KANGTI 1-56 B.Dhan Raj B.Kahi Nath ZPHS kandti 36 7 kangti 2-23 N Savitha Umakanth ZPHS Hanmanth rao pet 38 8 Kangti 22 Devisingh Ramavath Wangdhal ZPHS Chapta (K) 33 9 Wangdhal Mandal : KANGTI Village: NAGANPALLE 1-39 Pugula Sidda Reddy Pogula Kista Reddy ZPHS, Narayankhed 34 10 Naganpally 1-42 N.Laxaman Rao Shashe Rao ZPHS Regode 38 11 GARDEGAM 1-75/1 Manohar Rajappa ZPHSNiazampet 29 12 Naganpally 1of 304 Draft Electoral Roll of Medak-Nizamabad-Adilabad-Karimnagar Teachers Constituency of the A.P Legislative Council as published on 15-12-2012 Polling Station Number : ( 95 ) Kangti District: Medak - 17 MPCPS Kangti Sl.No. -
Name, Designation and Other Particulars of Public Information Officers
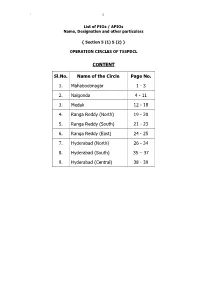
` 0 List of PIOs / APIOs Name, Designation and other particulars { Section 5 (1) 5 (2) } OPERATION CIRCLES OF TSSPDCL CONTENT Sl.No. Name of the Circle Page No. 1. Mahaboobnagar 1 - 3 2. Nalgonda 4 - 11 3. Medak 12 - 18 4. Ranga Reddy (North) 19 - 20 5. Ranga Reddy (South) 21 - 23 6. Ranga Reddy (East) 24 - 25 7. Hyderabad (North) 26 - 34 8. Hyderabad (South) 35 – 37 9. Hyderabad (Central) 38 - 39 ` 1 Name, Designation and other Particulars of Public Information Officers { Section 5 (1) 5 (2) } Mahaboobnagar Circle Sl. Name of the office Name of the PIO and Cell No. Name of the APIO and Cell No. No. District office Circle / Sri N.S.R.Murthy, DE/Technical,,Cell:9440813415, Sri B. Manikyalu, ADE/Tech., 1 Mahaboobnagar 08542-272946-272714,Fax 08542 - 272798 ,cell:9440813415, 08542-272730 G.Sudha Rani AE/Tech/D.O./MBNR,, 2 Division /Mahaboobnagar Venkataiah, DE/OP/MAHABUBNAGAR,, 9440813418 08542-273138 Sub-division, Sureshkumar, Sub-Er/MBNR(TOW),, 3 Srinivasa Chary, ADE/OP/MBNR(TOWN).,, 9440813431 Mahaboobnagar 08503-272926 Shanthi , Sub-Er./OP/TOWN-1, 4 Section Town-I/MBNR B.Ramesh Babu, AE/OP/TOWN-1,, 9440813457 08542-241666 Vacant, Sub-Er./OP/TOWN-2, 5 Section Town-II/MBNR Satyanna, AE/OP/TOWN-2,, 9491066205 08542-243666 6 Section /Town-III/MBNR M.Srinivas, AE/OP/TOWN-3,, 9440813459 K.Venkatesh/ Town-3,, 08542-243555 Ramakrishna, Sub-Er/MBNR(RURAL),, 7 Sub-division,MBNR Rural B.Srinivasulu, ADE/OP/MBNR(RURAL),, 9440813444 08542-273146 Chandra Shekar sub-eng I/C AE/OP/C&O/MBNR,, Chandra shekar sub-Er (I/C), 8 C&O/Section/MBNR 9440813890 Sub-Engineer, 9 Section/Koilkonda B. -

TRT - 2017 - ERSTWHILE MEDAK DISTRICT SGT (TELUGU MEDIUM ) -LOCAL BODY MGMT - VACANCIES No of UDISE New S
Page-1 of 12 TRT - 2017 - ERSTWHILE MEDAK DISTRICT SGT (TELUGU MEDIUM ) -LOCAL BODY MGMT - VACANCIES No of UDISE New S. No. Name of the School Mandal Posts CODE District Vacant 1 2 3 4 5 6 1 36170300201 P.S. Appajipally (T) ALLADURG 1 Medak 2 36170300103 P.S. Kaidampally ALLADURG 1 Medak 3 36170300902 P.S. Manikraj (T) ALLADURG 1 Medak 4 36170300301 P.S. Mupparam T ALLADURG 1 Medak 5 36170300301 P.S. Mupparam T ALLADURG 1 Medak 6 36170300101 P.S. Reddipally ALLADURG 1 Medak 7 36170300101 P.S. Reddipally ALLADURG 1 Medak 8 36170300102 P.S. Sitha Nagar ALLADURG 1 Medak 9 36170300102 P.S. Sitha Nagar ALLADURG 1 Medak 10 36170300110 PS.Gandlabavi T ALLADURG 1 Medak 11 36170300202 UPS Appajipally ALLADURG 1 Medak 12 36170300302 UPS Mupparam ALLADURG 1 Medak 13 36170300302 UPS Mupparam ALLADURG 1 Medak 14 36170300115 UPS Venkatraopet ALLADURG 1 Medak 15 36170902002 MPPS.Saju Thanda Chegunta 1 Medak 16 36171401302 MPPS ANTHARAM THANDA CHILIPCHED 1 Medak 17 36171400902 MPPS AZZAMARRI CHILIPCHED 1 Medak 18 36171400302 MPPS GIRIJA THANDA CHILIPCHED 1 Medak 19 36171400302 MPPS GIRIJA THANDA CHILIPCHED 1 Medak 20 36171400301 MPPS GOUTHAPUR THANDA CHILIPCHED 1 Medak 21 36171400301 MPPS GOUTHAPUR THANDA CHILIPCHED 1 Medak 22 36171400702 MPPS KOTHAKUNTA THANDA CHILIPCHED 1 Medak 23 36171400303 MPPS MALLAKKACHERU THANDA CHILIPCHED 1 Medak 24 36171401201 MPPS RAHEEMGUDA CHILIPCHED 1 Medak 25 36171400201 MPPS RAMDASGUDDA CHILIPCHED 1 Medak 26 36171400602 MPPS SAMLA THANDA CHILIPCHED 1 Medak 27 36171400501 MPPS SEELAMPALLY CHILIPCHED 1 Medak 28 36171400605 -

Aadhar Enrollment Centres
Branch Slno BranchName Region District Address of the centre Remarks State code 1 7110 Padra AshokNagar MAHABUBNAGAR PADRA,TEH.ACHAMPET, , , , working Telanagana 2 7143 Amangal AshokNagar MAHABUBNAGAR AMANGAL,TEH.KALWAKURTHY,509321, , , , working Telanagana PLOT No. 4 to 8, SURVEY No. 11, HYDERABAD X ROAD, 3 7194 Hyderabad X roads Kalwakurthy AshokNagar MAHABUBNAGAR KALWAKURTHY, KALWAKURTHY, 509324 working Telanagana 4 8115 Ashok Nagar AshokNagar Medak KUCHANPALLY,TEH.MEDAK,502110, , , , working Telanagana 5 8160 Gajwel AshokNagar Medak MAIN ROAD,GAJWEL,TEH.GAJWEL, , , , working Telanagana 6 4129 Aswaraopeta Bhadrachalam KHAMMAM ASWARAOPET, , , , working Telanagana 7 4144 Bandaru Gudem Bhadrachalam KHAMMAM MAIN RD,BANDARUGUDEM,507117, , , , working Telanagana 8 4158 Sujathanagar Bhadrachalam KHAMMAM 536/2,SUJATHANAGAR,TEH.KOTHAGUDEM, , , , working Telanagana 9 4160 Yellandu Bhadrachalam KHAMMAM H NO 5-1-125,NEAR NEW BUS STATION,YELLANDU,507123, , , , working Telanagana 10 4175 Sarapaka – 4175 Bhadrachalam KHAMMAM H.NO.1-117, , SARAPAKA, SARAPAKA, 507128 working Telanagana H. No. 5-35/6, MAHESWARI THEATER ROAD, LAXMIDEVIPALLI, 11 4190 Laxmidevipalli Bhadrachalam KHAMMAM LAXMIDEVIPALLI, 507101 working Telanagana 12 4125 Tirumalayapalem Khammam KHAMMAM THIRUMALAYAPALEM, , , , Under shifting Telanagana 11-4-100 WYRA RD,NEAR SOUTHERN AGENCIES,KHAMMAM., , , 13 4101 Khammam Khammam KHAMMAM , working Telanagana 14 4106 Kusumanchi Khammam KHAMMAM KUSUMANCHI,TEH.PALAIR, , , , working Telanagana H. No. 7-43, RAYAPATNAM ROAD, MADHIRA, MADHIRA, 15 -

State District Branch Address Centre Ifsc
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE A.N.REDDY NAGAR ANDHRA A N REDDY BR,NIRMAL,ANDHRA PRADESH ADILABAD NAGAR PRADESH NIRMAL ANDB0001972 8734243159 NONMICR 3-2-29/18D, 1ST CH.NAGAB FLOOR, AMBEDKAR HUSHANA ANDHRA CHOWK ADILABAD - M 08732- PRADESH ADILABAD ADILABAD 504 001 ADILABAD ANDB0000022 230766 TARA COMPLEX,MAIN ANDHRA ROAD,ASIFABAD,ADI 08733 PRADESH ADILABAD ASIFABAD LABAD DT - 504293 ASIFABAD ANDB0002010 279211 504011293 TEMPLE STREET, BASARA ADILABAD, ANDHRA ADILABAD, ANDHRA 986613998 PRADESH ADILABAD BASARA PRADESH-504104 BASAR ANDB0001485 1 Bazar Area, Bellampally , Adilabad G.Jeevan Reddy ANDHRA Dist - - 08735- PRADESH ADILABAD Bellampalli Bellampalli ADILABAD ANDB0000068 504251 2222115 ANDHRA BANK, BHAINSA BASAR P.SATYAN ROAD BHAINSA- ARAYANA - ANDHRA 504103 ADILABAD 08752- PRADESH ADILABAD BHAINSA DIST BHAINSA ANDB0000067 231108 D.NO 4-113/3/2,GOVT JUNIOR COLLEGE ROAD,NEAR BUS ANDHRA STAND,BOATH - 949452190 PRADESH ADILABAD BOATH 504305 BOATH ANDB0002091 1 MAIN ROAD,CHENNUR, ADILABAD DIST, ANDHRA CHENNUR, ANDHRA 087372412 PRADESH ADILABAD CHENNUR PRADESH-504201 CHINNOR ANDB0000098 36 9-25/1 BESIDE TANISHA GARDENS, ANDHRA DASNAPUR, PRADESH ADILABAD DASNAPUR ADILABAD - 504001 ADILABAD ANDB0001971 NO NONMICR ORIENT CEMENT WORKS CO, DEVAPUR,ADILABAD DIST, DEVAPUR, ANDHRA ANDHRA PRADESH- 08736 PRADESH ADILABAD DEVAPUR 504218 DEVAPUR ANDB0000135 240531 DOWEDPALLI, LXXETTIPET 08739- ANDHRA VILLAGE, GANDHI DOWDEPAL 233666/238 PRADESH ADILABAD DOWDEPALLI CHOWK LI ANDB0000767 222 H NO 1-171 VILL -
Redgram Outlook November 2020
Redgram Outlook, November 2020 Redgram Outlook – November 2020 Redgram is commonly known as Tur or Arhar in India and is the second important pulse crop in the country after gram (chana). The ability of redgram to produce high economic yields under soil moisture deficit makes it an important crop in rainfed and dry land agriculture. World major redgram producing countries are India (37.50 lakh tonnes), Myanmar (6.76 lakh tonnes), Malawi (4.34 lakh tonnes), Tanzania (3.15 lakh tonnes) and Haiti (0.87 lakh tonnes). Area under redgram reported during 2020-21 was 48.24 lakh ha (119.20 lakh acres) as against 45.45 lakh ha (112.31 lakh acres) during the same period in 2019-20. In India, major redgram producing states are Karnataka 12.80 lakh ha (31.63 lakh acres), Maharashtra 12.46 lakh ha (30.79 lakh acres), Telangana 4.34 lakh ha (10.72 lakh acres), Madhya Pradesh 4.12 lakh ha (10.18 lakh acres) and Uttar Pradesh 3.53 lakh ha (8.72 lakh acres). According to Government 1st advance estimates, all India redgram production in 2020-21 is at 4.04 million tonnes. 4.87 4.29 4.04 3.83 3.32 3.02 3.17 2.81 2.65 2.56 2011-12 2012-13 2013-14 2014-15 2015-16 2016-17 2017-18 2018-19 2019-20 2020-21* Source: Directorate of Economics and Statistics (DES). Figure 1: Production of Redgram in India (in million tonnes) Agricultural Market Intelligence Centre, PJTSAU Page 1 Redgram Outlook, November 2020 Table 1: Redgram Domestic Supply & Demand (in lakh tonnes) 2020-21* 2018-19 2019-20 Opening Stocks 8.49 6.66 7.71 Production 33.20 38.30 40.4 Imports 5.30 5.00 5 Total Supply 46.99 49.96 53.11 Exports 0.33 0.25 0.4 Consumption 40.00 42.00 43.5 Total Demand 40.33 42.25 43.9 Ending Stocks 6.66 7.71 9.21 Source: www.agriwatch.com *Estimated based on 1st Advance Estimates of Government of India The major markets for this crop in Telangana are Badepalli, Devarakadra, Gadwal, Mahabubnagar, Narayanpet, Sadasivpet, Zaheerabad, Suryapet, Tandur and Warangal. -
TELANGANA STATE INFORMATION COMMISSION (Under Right To
TELANGANA STATE INFORMATION COMMISSION (Under Right to Information Act, 2005) Samachara Hakku Bhavan, D.No.5-4-399, ‘4’ Storied Commercial Complex, Housing Board Building, Mojam Jahi Market, Hyderabad – 500 001. Phone Nos: 040-24743399 (O); 040-24740592(F) Appeal No:12500/SIC-BM/2018, Order dated:26-04-2019 Appellant : Sri B. Dasaratha Reddy, S/o Sanga Reddy, H.No.3-4-42, Tilak Road, Sadasivpet, Sangareddy District-502291. Respondents : The Public Information Officer (U/RTI Act,2005) / O/o the Deputy Tahsildar, Sadasivpet Mandal, Sadasivpet, Sangareddy District-502291. : The First Appellate Authority (U/RTI Act, 2005) / The Deputy Tahsildar, Sadasivpet Mandal, Sadasivpet, Sangareddy District- 502291. ORDER Sri. B. Dasaratha Reddy has filed 2nd appeal dated 05-09-2018 which was received by this Commission on 05-09-2018 for not getting the information sought by him from the Public Information Officer/ O/o the Deputy Tahsildar, Sadasivpet Mandal, Sangareddy District and 1st Appellate Authority/ the Deputy Tahsildar, Sadasivpet Mandal, Sangareddy District. The brief facts of the case as per the appeal and other records received along with it are that the appellant herein filed an applications dated: 30-04-2018 before the PIO requesting to furnish the information under Sec. 6(1) of the RTI Act, 2005 on the following pointsTSIC mentioned: Stating that the appellant did not get any response from the Public Information Officer, he filed 1st appeals dated 19-06-2018 before the 1st Appellate Authority requesting him to furnish the information sought by him u/s 19(1) of the RTI Act, 2005. The Public Information Officer through Lr.No. -

Executive Summary
PLP 2016-17 | Medak Executive Summary This document, the Potential linked credit plan (PLP) for the district, Medak, aims to transform rural economy of the district by assessing credit requirements of various sectors and to provide a road map to banks for extending credit to these sectors. The document is prepared through consultative credit planning with vision for infrastructure linkage to achieve accelerated growth and creating sustainable livelihood opportunities by ensuring food security, inclusive growth and effective credit dispensation. The theme for the PLP 2016-17 is “Accelerating the pace of capital formation in agriculture and allied sector”. The PLP has been redesigned with the objective of making it a meaningful link between development planning and credit planning process, leading to action planning. The plan which was prepared for five years, co-terminus with the XII Five Year Plan period i.e. from 2012-13 to 2016-17 and potentials had been worked out for each sector / sub sector that could be tapped with institutional credit during the XII year plan period, has suitably been modified taking into account current developments, especially revision in priority sector guidelines and policy initiatives of government. Also, keeping in view the national priority, the share of term loan for agriculture and allied activities is kept at 26%(`1032.44 crore) of the total potential (`3968.84 crore) under Agriculture sector estimated for the year 2016-17. The geographical area of the district is 9699 sq. kms and it is divided into 3 revenue divisions viz., Medak, Siddipet and Sangareddy. The population of the district as per census, 2011 is 30.33 lakh with 76% (23.05 lakh) people living in rural areas.