Microbial Diversity in Alpine Tundra Soils Correlates with Snow Cover Dynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Expression of NOD2, NLRP3 and NLRC5 and Renal Injury in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis
Wang et al. J Transl Med (2019) 17:197 https://doi.org/10.1186/s12967-019-1949-5 Journal of Translational Medicine RESEARCH Open Access The expression of NOD2, NLRP3 and NLRC5 and renal injury in anti-neutrophil cytoplasmic antibody-associated vasculitis Luo‑Yi Wang1,2,3, Xiao‑Jing Sun1,2,3, Min Chen1,2,3* and Ming‑Hui Zhao1,2,3,4 Abstract Background: Nucleotide‑binding oligomerization domain (NOD)‑like receptors (NLRs) are intracellular sensors of pathogens and molecules from damaged cells to regulate the infammatory response in the innate immune system. Emerging evidences suggested a potential role of NLRs in anti‑neutrophil cytoplasmic antibody (ANCA)‑associated vasculitis (AAV). This study aimed to investigate the expression of nucleotide‑binding oligomerization domain con‑ taining protein 2 (NOD2), NOD‑like receptor family pyrin domain containing 3 (NLRP3) and NOD‑like receptor family CARD domain containing 5 (NLRC5) in kidneys of AAV patients, and further explored their associations with clinical and pathological parameters. Methods: Thirty‑four AAV patients in active stage were recruited. Their renal specimens were processed with immu‑ nohistochemistry to assess the expression of three NLRs, and with double immunofuorescence to detect NLRs on intrinsic and infltrating cells. Analysis of gene expression was also adopted in cultured human podocytes. The associa‑ tions between expression of NLRs and clinicopathological parameters were analyzed. Results: The expression of NOD2, NLRP3 and NLRC5 was signifcantly higher in kidneys from AAV patients than those from normal controls, minimal change disease or class IV lupus nephritis. These NLRs co‑localized with podocytes and infltrating infammatory cells. -

Striking the Right Balance in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis
Striking the Right Balance in Anti-neutrophil Cytoplasmic Antibody-Associated Vasculitis This symposium took place on 4th June 2021, as part of the European Alliance of Associations for Rheumatology (EULAR) virtual congress Speakers: Benjamin Terrier,¹ Joanna Robson,² Bernhard Hellmich³ 1. University of Paris and Hôpital Cochin, France 2. University of the West of England and Bristol Royal Infirmary, UK 3. University of Tübingen, Germany Disclosure: Terrier has been an advisory board member and/or received consulting fees/ travel expenses from AstraZeneca, Chugai, Grifols, GlaxoSmithKline, Janssen, LFB, Octapharma, Roche, and Vifor Pharma. Robson has received speaker’s fees from Roche and Vifor Pharma; and research support from Vifor Pharma. Hellmich has been an investigator in clinical trials for Ab2Bio, AbbVie, AstraZeneca, Bristol- Myers Squibb, Chemocentrix, GlaxoSmithKline, InflaRx, Kiniksa, Nippon Kayaku, Novartis, Roche, and Sanofi. He has acted as a consultant, advisory board member, and/or lecturer for AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Chugai, GlaxoSmithKline, InflaRx, Novartis, Pfizer, Roche, and Vifor Pharma. He is also a member of the Guideline Committees for European Alliance of Associations for Rheumatology (EULAR) and the German Society of Rheumatology (DGRh). Acknowledgements: Writing assistance was provided by Helen Boreham. Support: The publication of this article was funded by Vifor Pharma. The views and opinions expressed are those of the presenters. Content was reviewed by Vifor Pharma for medical accuracy. Citation: Rheumatol. 2021;8[1]:43-50. Meeting Summary Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) causes irreversible short- and long-term damage to vital organs, particularly the kidneys and lungs. Current standard of care (SOC) for AAV, of which glucocorticoids (GC) are a lynchpin, has a number of important limitations: responses to therapy are variable, some patients fail to achieve and sustain remission, and treatment related adverse events (AE) are common. -
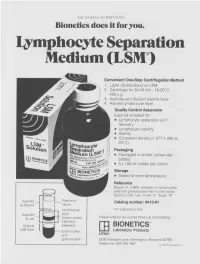
Lymphocyte Separation Medium (LSM
THE JOURNAL OF IMMUNOLOGY Bionetics does it for you. Lymphocyte Separation Medium (LSM wenient One-Step Centrifugation Method _ayer diluted blood on LSM. 2,entrifuge for 30-40 min., 18-20°C, ~.00 x g. ~,spirate and discard plasma layer -larvest lymphocyte layer. Quality Control Assurance Each lot is tested for: • Lymphocyte separation and recovery. • Lymphocyte viability. • Sterility. • Consistent density (1.077-1.080 at 20°C). Packaging • Packaged in amber, screw-cap bottles. • 5 x 100 ml bottles per carton. Storage. • Stored at room temperature. Reference Boyum, A. (1968): Isolation of mononuclear cells and granulocytes from human blood. Scand J. Clin. Lab. Invest. 21, Suppl. 97. Aspirate I IC[OI I IC~ LJI Catalog number: 8410-01 & discard serum Lymphocyte For Laboratory Use Aspirate layer & use (mononuclear Please write for our current Price List and Catalog. cells and Original platelets) ITi BIONETICS° LSM layer Erythrocytes Laboratory Products and Litton granulocytes 5516 Nicholson Lane, Kensington, Maryland 20795 Telephone: (301) 881-1557 1979 Litton Bionetics, tnc Get the most out of your high quality cytotoxic antibodies with LOW-TOX-M RABBIT COMPLEMENT LOW TOXICITY HIGH ACTIVITY Presentation: CL 3051 5 x 1 ml, lyophilized $30.00 When it comes to COMPLEMENT... come to CEDARLANE Direct orders or inquiries to: UNITED STATES: WORLDWIDE EXCEPT U.S. ,4 C~L CEDARLANE ACCURATE CHEMICAL & LABORATO RI ES SCIENTIFIC CORPORATION LIMITED 5516-8TH LINE, R.R. 2 28 TEC STREET, HICKSVtLLE, N.Y. 11801 HORNBY, ONTARIO, CANADA LOP 1E0 Telephone -

Induction of Protein Citrullination and Auto-Antibodies Production In
www.nature.com/scientificreports OPEN Induction of protein citrullination and auto-antibodies production in murine exposed to nickel Received: 11 November 2015 Accepted: 21 December 2017 nanomaterials Published: xx xx xxxx Bashir M. Mohamed1,7, Noreen T. Boyle2,3, Anja Schinwald4, Bruno Murer5, Ronan Ward6, Omar K. Mahfoud1, Tatsiana Rakovich1, Kieran Crosbie-Staunton1, Steven G. Gray 7, Ken Donaldson4, Yuri Volkov1,2 & Adriele Prina-Mello 1,2 Citrullination, or the post-translational deimination of polypeptide-bound arginine, is involved in several pathological processes in the body, including autoimmunity and tumorigenesis. Recent studies have shown that nanomaterials can trigger protein citrullination, which might constitute a common pathogenic link to disease development. Here we demonstrated auto-antibody production in serum of nanomaterials-treated mice. Citrullination-associated phenomena and PAD levels were found to be elevated in nanomaterials -treated cell lines as well as in the spleen, kidneys and lymph nodes of mice, suggesting a systemic response to nanomaterials injection, and validated in human pleural and pericardial malignant mesothelioma (MM) samples. The observed systemic responses in mice exposed to nanomaterials support the evidence linking exposure to environmental factors with the development of autoimmunity responses and reinforces the need for comprehensive safety screening of nanomaterials. Furthermore, these nanomaterials induce pathological processes that mimic those observed in Pleural MM, and therefore require further investigations into their carcinogenicity. Citrullination is involved in several pathological processes in the body, including autoimmunity and tumor- igenesis. Citrullinated proteins are generated by a post-translational deimination or demethylimination of polypeptide-bound arginine by a family of Ca2+-dependent enzyme peptidylarginine deiminase (PAD)1. -

Heritability of Autoantibody Levels in a Twin Population
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2009 Heritability of Autoantibody Levels in a Twin Population Amal Rastogi Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Periodontics and Periodontology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/1854 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. School of Dentistry Virginia Commonwealth University This is to certify that the thesis prepared by Amal Rastogi, DMD, PhD entitled HERITABILITY OF AUTOANTIBODY LEVELS IN A TWIN POPULATION has been approved by his committee as satisfactory completion of the thesis requirement for the degree of Master of Science in Dentistry. John Gunsolley, DDS, MS, Professor, Department of Periodontics, VCU, School of Dentistry Harvey Schenkein, DDS, PhD, Chair, Department of Periodontics, VCU, School of Dentistry Robert Sabatini, DDS, MS, Assistant Professor, Department of Periodontics, VCU, School of Dentistry Thomas Waldrop, DDS, MS, Graduate Director, Department of Periodontics, VCU, School of Dentistry Harvey Schenkein, DDS, PhD, Chair, Department of Periodontics, VCU, School of Dentistry Laurie Carter, DDS, PhD, Director of Advanced Dental Education,VCU, School of Dentistry Dr. F. Douglas Boudinot, Dean of the Graduate School, VCU June 29, 2009 © Amal Rastogi, DMD, PhD 2009 All Rights Reserved 2 HERITABILITY OF AUTOANTIBODY LEVELS IN A TWIN POPULATION A thesis submitted in partial fulfillment of the requirements for the degree of MSD at Virginia Commonwealth University. -

Antibody (ANCA)-Associated Vasculitis Avacopan Introduction
CO-1 Avacopan for the Treatment of Anti-Neutrophil Cytoplasmic Auto- antibody (ANCA)-Associated Vasculitis ChemoCentryx, Inc. Arthritis Advisory Committee May 6, 2021 CO-2 Avacopan Introduction Thomas J. Schall, Ph.D. President, Chief Executive Officer ChemoCentryx, Inc. CO-3 Avacopan: First-in-Class, Targeted Therapy for ANCA-Associated Vasculitis ANCA-associated vasculitis is rare, severe, and often fatal autoimmune disease Anti-neutrophil cytoplasmic auto-antibodies (ANCA) involved in pathogenesis Inflammation of small vessels, can affect any organ Commonly affects kidneys Glucocorticoid treatment associated with significant toxicities Despite current therapies, > 1 in 10 patients die within first year of diagnosis1,2 1. Heijl et al., 2017; 2. Little et al., 2010 CO-4 Central Role of C5a in Pathogenesis of ANCA-Associated Vasculitis Jennette and Falk, 2014 CO-5 Avacopan: Highly Potent and Selective C5aR Inhibitor Avacopan avoids long-term 1 biological consequences of ‘upstream’ complement inhibition 1 Does not block C5b-9 production; leaves host defense mechanism C5aC5a AntibodiesAntibodies 2 membrane attack complex (MAC) in place C6-C9C6-C9 AvacopanAvacopan Preserves beneficial 3 functions of C5L2 pathway C5aR Leukocyte migration and signaling Leukocyte trafficking, Cell lysis migration and (i.e., Neisseria control activation Beneficial Targets ‘downstream' anti-inflammatoryanti-inflammatory 4 effect complement pathway Adaptive Phagocytosis 4 3 2 signaling and clearance CO-6 Avacopan in ANCA-Associated Vasculitis Pirow Bekker, MD, PhD Clinical Lead Avacopan Clinical Development Program ChemoCentryx, Inc. CO-7 Avacopan Proposed Indication and Dose Proposed Indication …for the treatment of adult patients with anti-neutrophil cytoplasmic auto-antibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis and microscopic polyangiitis). -

Degradation of Neutrophil Extracellular Traps Is Decreased in Patients with Antiphospholipid Syndrome J
Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome J. Leffler1, L. Stojanovich2, Y. Shoenfeld3, G. Bogdanovic2, R. Hesselstrand4, A.M. Blom1 1Dept. of Laboratory Medicine, Section of Medical Protein Chemistry, Lund University, Malmö, Sweden; 2Dept. of Internal Medicine, “Bezhanijska Kosa” University Medical Center, Belgrade, Serbia; 3Zabludowicz Center for Autoimmune Diseases, Sheba Medical Center, Tel Aviv, Israel; 4Dept. of Clinical Sciences, Section of Rheumatology, Lund University, Lund, Sweden. Abstract Objective A decreased ability to degrade neutrophil extracellular traps (NETs) is seen in a subgroup of patients with systemic lupus erythematosus (SLE) and correlates with the presence of autoantibodies. Antiphospholipid syndrome (APS) can develop secondary to SLE or as a primary disease. In the current study we investigated the ability of sera from patients with APS to degrade NETs. The presence of antibodies against NETs and neutrophil remnants were also determined in the same patients. Methods In the study, 106 patients with APS (73 primary and 33 secondary), 76 patients with systemic sclerosis (SSc) and 77 healthy donors as control samples were included. NETs generated from neutrophils isolated from healthy volunteers were incubated with patient sera, followed by measurement of degraded NETs or deposited IgG. Results Sera of APS patients had a decreased ability to degrade NETs compared to healthy controls, with no difference between primary and secondary APS. Sera from SSc patients did not differ significantly from healthy controls in the ability to degrade NETs. A decreased degradation of NETs correlated weakly to increased levels of antibodies against NETs/ neutrophil remnants in patients with primary APS, but stronger in patients with secondary APS. -

Belimumab (Benlysta®)
Policy Medical Policy Manual Approved Revision: Do Not Implement until 8/31/21 Belimumab (Benlysta®) NDC CODE(S) 49401-0101-XX BENLYSTA 120MG Solution Reconstituted (GLAXO SMITH KLINE) 49401-0102-XX BENLYSTA 400MG Solution Reconstituted (GLAXO SMITH KLINE) DESCRIPTION Belimumab is a human IgG1 monoclonal antibody specific for soluble human B lymphocyte stimulator protein (BLyS), a B cell survival factor. It is produced by recombinant DNA technology in a mammalian cell expression system. Belimumab does not bind to B cells directly but blocks access of soluble BLyS to its receptors on B cells. This inhibits the survival of B cells and reduces the differentiation of B cells into immunoglobulin-producing plasma cells. Treatment with belimumab leads to reductions in circulating CD19+, CD20+, naïve and activated B cells along with plasmacytoid cells and the systemic lupus erythematosus (SLE) B-cell subset. POLICY Belimumab for the treatment of the following is considered medically necessary if the medical appropriateness criteria are met. (See Medical Appropriateness below.) o Systemic Lupus Erythematosus (SLE) o Lupus Nephritis Belimumab or the treatment of other conditions/diseases, including, but not limited to, Active Central Nervous System Lupus is considered investigational. MEDICAL APPROPRIATENESS INITIAL APPROVAL CRITERIA Patient is at least 18 years of age (unless otherwise specified); AND Universal Criteria Patient must not have an active infection; AND Patient has not received a live vaccine within 30 days before starting or -

Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens
ARTHRITIS & RHEUMATOLOGY Vol. 67, No. 12, December 2015, pp 3135–3145 DOI 10.1002/art.39313 VC 2015 The Authors. Arthritis & Rheumatolgy published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited. Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid Julia Spengler,1 Bozo Lugonja,1 A. Jimmy Ytterberg,2 Roman A. Zubarev,3 Andrew J. Creese,4 Mark J. Pearson,1 Melissa M. Grant,4 Michael Milward,4 Karin Lundberg,2 Christopher D. Buckley,5 Andrew Filer,6 Karim Raza,5 Paul R. Cooper,4 Iain L. Chapple,4 and Dagmar Scheel-Toellner1 Objective. In the majority of patients with rheu- citrullinated autoantigens that are generated by peptidy- matoid arthritis (RA), antibodies specifically recognize larginine deiminases (PADs). Neutrophils express high levels of PAD and accumulate in the synovial fluid (SF) The views expressed in this publication are those of the of RA patients during disease flares. This study was authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health. undertaken to test the hypothesis that neutrophil cell Supported by the European Union Seventh Framework Pro- death, induced by either NETosis (extrusion of genomic gramme (project Gums and Joints; HEALTH-2010-261460 and project DNA–protein complexes known as neutrophil extracellu- EuroTEAM; HEALTH-F2-2012-305549) and by the NIHR Wellcome Trust Clinical Research Facility at University Hospitals Birmingham lar traps [NETs]) or necrosis, can contribute to produc- NHS Foundation Trust. -

Defining Essential Elements and Genetic Interactions of the Yeast
Downloaded from rnajournal.cshlp.org on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press 1 Defining essential elements and genetic interactions of the yeast Lsm2-8 ring and demonstration that essentiality of Lsm2-8 is bypassed via overexpression of U6 snRNA or the U6 snRNP subunit Prp24 Allen J. Roth1, Stewart Shuman2, and Beate Schwer1 1 Microbiology and Immunology Department, Weill Cornell Medical College, New York, NY 10065; and 2 Molecular Biology Program, Sloan-Kettering Institute, New York, NY 10065 correspondence: [email protected]; [email protected] Downloaded from rnajournal.cshlp.org on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press 2 ABSTRACT A seven-subunit Lsm2-8 protein ring assembles on the U-rich 3' end of the U6 snRNA. A structure-guided mutational analysis of the Saccharomyces cerevisiae Lsm2-8 ring affords new insights to structure-function relations and genetic interactions of the Lsm subunits. Alanine scanning of 39 amino acids comprising the RNA-binding sites or inter-subunit interfaces of Lsm2, Lsm3, Lsm4, Lsm5, and Lsm8 identified only one instance of lethality (Lsm3-R69A) and one severe growth defect (Lsm2-R63A), both involving amino acids that bind the 3'-terminal UUU trinucleotide. All other Ala mutations were benign with respect to vegetative growth. Tests of 235 pairwise combinations of benign Lsm mutants identified six instances of inter-Lsm synthetic lethality and 45 cases of non-lethal synthetic growth defects. Thus, Lsm2-8 ring function is buffered by a network of internal genetic redundancies. A salient finding was that otherwise lethal single-gene deletions lsm2∆, lsm3∆, lsm4∆, lsm5, and lsm8∆ were rescued by overexpression of U6 snRNA from a high-copy plasmid. -

ZEISS LSM 800 with Airyscan Your Compact Confocal Power Pack
Product Information ZEISS LSM 800 with Airyscan Version 1.0 Your Compact Confocal Power Pack Your Compact Confocal Power Pack › In Brief Confocal imaging demands the very best imaging quality. With LSM 800 you are choosing a flexible and compact confocal laser scanning microscope, complete › The Advantages with highly sensitive GaAsP detector technology and fast linear scanning. › The Applications Add Airyscan, the revolutionary detection concept from ZEISS, and you will gain › The System 1.7× higher resolution in all three dimensions – resulting in a 5× smaller confocal volume. And you will be pushing sensitivity beyond the limits of all conventional › Technology and Details confocals. › Service Click here to view this video LSM 800 is your entry into the world of high-end confocal imaging. Simply decide which options your system needs today, then upgrade in the future as Mouse retina. Mueller cells stained for RFP (red), Amacrine cells stained for Chat (green), Cone photo receptors stained for mCar your needs grow. (white) and DNA stained with Hoechst (blue); Sample: courtesy of B. Roska, Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland See for yourself how LSM 800 with Airyscan will increase your productivity. Book a hands-on demonstration in one of our ZEISS Microscopy Labs now. >> www.zeiss.com/lsm800 2 Simpler. More Intelligent. More Integrated. Perfectly Tailored to Your Needs Use Open Interfaces to Extend Your System Your Compact System for High-end › In Brief Confocal Imaging › The Advantages With up to three highly sensitive GaAsP detectors Give your lab or multi-user facility the full benefits LSM 800 makes excellent economic sense: and fast linear scanning, LSM 800 brings you of integrated incubation solutions and state-of- an affordable system with an attractive price / › The Applications higher productivity and throughput, greater the-art Axiocams. -

LSM 510 in Live Cell Imaging Welcome
LSM 510 in Live Cell Imaging Welcome Dr. Jörg Lindenau Training, Application and Support Center -TASC- 20.04.2004 LSM 510 in Live Cell Imaging Content The Subject of Interest Scanning Strategies for fast Image Acquisition Multicolor Labeling Emission and Excitation Crosstalk Spectral dimension - Emission Fingerprinting - Excitation Fingerprinting - Online Fingerprinting Applications - Quantitative FRET Imaging - FRAP and FLIP - Photoactivation & Photoconversion Zeiss features for live cell imaging Dr. Jörg Lindenau Training, Application and Support Center -TASC- 20.04.2004 LSM 510 in Live Cell Imaging The Subject of Interest In fact Cells are highly dynamic Structures. The decision of a cell to proliferate or to underwent cellular suicide depends on dynamic analyses of a complex information network. Dr. Jörg Lindenau Training, Application and Support Center -TASC- 20.04.2004 LSM 510 in Live Cell Imaging Starting with the details – fundamental Questions • Which Ligand binds to the receptor? • Is molecule A changing the conformation of the complex of the molecules B and C? • How fast can a membrane associated protein reach its target structure in the nucleus? • Is there a binding secuence for that protein at DNA? What happens after binding? • Which nerve cell is responsible to react after a visual stimulus? • Which cells of an embryo are the progenitors liver cells? • How can the malaria germ enter a blood cell? Dr. Jörg Lindenau Training, Application and Support Center -TASC- 20.04.2004 LSM 510 in Live Cell Imaging What are the specimens? Specimen Required equipment Cell culture Cell culture lab, cell incubation equipment at the microscope Tissue culture, tissue co cultures or Tissue culture lab, advanced incubation combinations of cell and tissue cultures equipment at the microscope (O2 control), with anorganic materials micro manipulation Living animals Animal housing, surgery equipment, special holding frames, vital function monitoring, micro manipulation Dr.