Antibiotic Susceptibility Profiles of Lactic Acid Bacteria from The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Expression of NOD2, NLRP3 and NLRC5 and Renal Injury in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis
Wang et al. J Transl Med (2019) 17:197 https://doi.org/10.1186/s12967-019-1949-5 Journal of Translational Medicine RESEARCH Open Access The expression of NOD2, NLRP3 and NLRC5 and renal injury in anti-neutrophil cytoplasmic antibody-associated vasculitis Luo‑Yi Wang1,2,3, Xiao‑Jing Sun1,2,3, Min Chen1,2,3* and Ming‑Hui Zhao1,2,3,4 Abstract Background: Nucleotide‑binding oligomerization domain (NOD)‑like receptors (NLRs) are intracellular sensors of pathogens and molecules from damaged cells to regulate the infammatory response in the innate immune system. Emerging evidences suggested a potential role of NLRs in anti‑neutrophil cytoplasmic antibody (ANCA)‑associated vasculitis (AAV). This study aimed to investigate the expression of nucleotide‑binding oligomerization domain con‑ taining protein 2 (NOD2), NOD‑like receptor family pyrin domain containing 3 (NLRP3) and NOD‑like receptor family CARD domain containing 5 (NLRC5) in kidneys of AAV patients, and further explored their associations with clinical and pathological parameters. Methods: Thirty‑four AAV patients in active stage were recruited. Their renal specimens were processed with immu‑ nohistochemistry to assess the expression of three NLRs, and with double immunofuorescence to detect NLRs on intrinsic and infltrating cells. Analysis of gene expression was also adopted in cultured human podocytes. The associa‑ tions between expression of NLRs and clinicopathological parameters were analyzed. Results: The expression of NOD2, NLRP3 and NLRC5 was signifcantly higher in kidneys from AAV patients than those from normal controls, minimal change disease or class IV lupus nephritis. These NLRs co‑localized with podocytes and infltrating infammatory cells. -

Utilization of Lactic Acid in Human Myotubes and Interplay with Glucose and Fatty Acid Metabolism Received: 12 January 2018 Jenny Lund1, Vigdis Aas2, Ragna H
www.nature.com/scientificreports OPEN Utilization of lactic acid in human myotubes and interplay with glucose and fatty acid metabolism Received: 12 January 2018 Jenny Lund1, Vigdis Aas2, Ragna H. Tingstad2, Alfons Van Hees2 & Nataša Nikolić1 Accepted: 11 June 2018 Once assumed only to be a waste product of anaerobe glycolytic activity, lactate is now recognized as Published: xx xx xxxx an energy source in skeletal muscles. While lactate metabolism has been extensively studied in vivo, underlying cellular processes are poorly described. This study aimed to examine lactate metabolism in cultured human myotubes and to investigate efects of lactate exposure on metabolism of oleic acid and glucose. Lactic acid, fatty acid and glucose metabolism were studied in myotubes using [14C(U)] lactic acid, [14C]oleic acid and [14C(U)]glucose, respectively. Myotubes expressed both the MCT1, MCT2, MCT3 and MCT4 lactate transporters, and lactic acid was found to be a substrate for both glycogen synthesis and lipid storage. Pyruvate and palmitic acid inhibited lactic acid oxidation, whilst glucose and α-cyano-4-hydroxycinnamic acid inhibited lactic acid uptake. Acute addition of lactic acid inhibited glucose and oleic acid oxidation, whereas oleic acid uptake was increased. Pretreatment with lactic acid for 24 h did not afect glucose or oleic acid metabolism. By replacing glucose with lactic acid during the whole culturing period, glucose uptake and oxidation were increased by 2.8-fold and 3-fold, respectively, and oleic acid oxidation was increased 1.4-fold. Thus, lactic acid has an important role in energy metabolism of human myotubes. Lactate is produced from glucose through glycolysis and the conversion of pyruvate by lactate dehydrogenase (LDH). -

Lactate Like Fluconazole Reduces Ergosterol Content in the Plasma Membrane and Synergistically Kills Candida Albicans
International Journal of Molecular Sciences Article Lactate Like Fluconazole Reduces Ergosterol Content in the Plasma Membrane and Synergistically Kills Candida albicans Jakub Suchodolski 1, Jakub Muraszko 1 , Przemysław Bernat 2 and Anna Krasowska 1,* 1 Department of Biotransformation, Faculty of Biotechnology, University of Wrocław, 50-383 Wrocław, Poland; [email protected] (J.S.); [email protected] (J.M.) 2 Department of Industrial Microbiology and Biotechnology, Faculty of Biology and Environmental Protection, University of Łód´z,90-237 Łód´z,Poland; [email protected] * Correspondence: [email protected] Abstract: Candida albicans is an opportunistic pathogen that induces vulvovaginal candidiasis (VVC), among other diseases. In the vaginal environment, the source of carbon for C. albicans can be either lactic acid or its dissociated form, lactate. It has been shown that lactate, similar to the popular antifungal drug fluconazole (FLC), reduces the expression of the ERG11 gene and hence the amount of ergosterol in the plasma membrane. The Cdr1 transporter that effluxes xenobiotics from C. albicans cells, including FLC, is delocalized from the plasma membrane to a vacuole under the influence of lactate. Despite the overexpression of the CDR1 gene and the increased activity of Cdr1p, C. albicans is fourfold more sensitive to FLC in the presence of lactate than when glucose is the source of carbon. We propose synergistic effects of lactate and FLC in that they block Cdr1 activity by delocalization due to changes in the ergosterol content of the plasma membrane. Citation: Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. Lactate Keywords: Candida albicans; lactate; fluconazole; ergosterol; Cdr1 Like Fluconazole Reduces Ergosterol Content in the Plasma Membrane and Synergistically Kills Candida albicans. -

United States Patent (10) Patent No.: US 8.450,378 B2 Snyder Et Al
USOO8450378B2 (12) United States Patent (10) Patent No.: US 8.450,378 B2 Snyder et al. (45) Date of Patent: *May 28, 2013 (54) ANTIVIRAL METHOD 5,944.912 A 8/1999 Jenkins ........................... 134/40 5,951,993 A 9, 1999 Scholz. ....... ... 424/405 (75) Inventors: Marcia Snyder, Stow, OH (US); David 6,022,5515,965,610 A 10/19992/2000 JampaniModak et ..... al. ... 424/405514,494 R. Macinga, Stow, OH (US); James W. 6,025,314. A 2/2000 Nitsch ... ... 510,221 Arbogast, Bath, OH (US) 6,034,133. A 3/2000 Hendley . 514,573 6,080,417 A 6/2000 Kramer ......................... 424/405 (73) Assignee: GOJO Industries, Inc., Akron, OH 6,090,395 A 7/2000 ASmuS (US) 6,107,261 A 8/2000 Taylor ........................... 510,131 6,110,908 A 8/2000 Guthery . 514,188 (*) Notice: Subject to any disclaimer, the term of this 8:35, A 858 Flying . patent is extended or adjusted under 35 6,183,766 B1 2/2001 Sine et al. ... 424/405 U.S.C. 154(b) by 716 days. 6,204.230 B1 3/2001 Taylor ........ ... 510,131 6,294, 186 B1 9/2001 Beerse et al. ... 424/405 This patent is Subject to a terminal dis- 6,319,958 B1 1 1/2001 Johnson ..... 514,739 claimer. 6,326,430 B1 12/2001 Berte ..... 524,555 6,352,701 B1 3/2002 Scholz. ... ... 424/405 (21) Appl. No.: 12/189,139 6,423,329 B1 7/2002 Sine et al. .. ... 424/405 6,436,885 B2 8/2002 Biedermann . ... 510,131 6,468,508 B1 10/2002 Laughlin .. -

Striking the Right Balance in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis
Striking the Right Balance in Anti-neutrophil Cytoplasmic Antibody-Associated Vasculitis This symposium took place on 4th June 2021, as part of the European Alliance of Associations for Rheumatology (EULAR) virtual congress Speakers: Benjamin Terrier,¹ Joanna Robson,² Bernhard Hellmich³ 1. University of Paris and Hôpital Cochin, France 2. University of the West of England and Bristol Royal Infirmary, UK 3. University of Tübingen, Germany Disclosure: Terrier has been an advisory board member and/or received consulting fees/ travel expenses from AstraZeneca, Chugai, Grifols, GlaxoSmithKline, Janssen, LFB, Octapharma, Roche, and Vifor Pharma. Robson has received speaker’s fees from Roche and Vifor Pharma; and research support from Vifor Pharma. Hellmich has been an investigator in clinical trials for Ab2Bio, AbbVie, AstraZeneca, Bristol- Myers Squibb, Chemocentrix, GlaxoSmithKline, InflaRx, Kiniksa, Nippon Kayaku, Novartis, Roche, and Sanofi. He has acted as a consultant, advisory board member, and/or lecturer for AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Chugai, GlaxoSmithKline, InflaRx, Novartis, Pfizer, Roche, and Vifor Pharma. He is also a member of the Guideline Committees for European Alliance of Associations for Rheumatology (EULAR) and the German Society of Rheumatology (DGRh). Acknowledgements: Writing assistance was provided by Helen Boreham. Support: The publication of this article was funded by Vifor Pharma. The views and opinions expressed are those of the presenters. Content was reviewed by Vifor Pharma for medical accuracy. Citation: Rheumatol. 2021;8[1]:43-50. Meeting Summary Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) causes irreversible short- and long-term damage to vital organs, particularly the kidneys and lungs. Current standard of care (SOC) for AAV, of which glucocorticoids (GC) are a lynchpin, has a number of important limitations: responses to therapy are variable, some patients fail to achieve and sustain remission, and treatment related adverse events (AE) are common. -
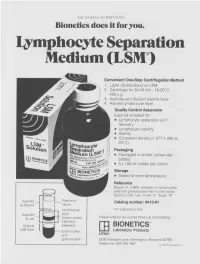
Lymphocyte Separation Medium (LSM
THE JOURNAL OF IMMUNOLOGY Bionetics does it for you. Lymphocyte Separation Medium (LSM wenient One-Step Centrifugation Method _ayer diluted blood on LSM. 2,entrifuge for 30-40 min., 18-20°C, ~.00 x g. ~,spirate and discard plasma layer -larvest lymphocyte layer. Quality Control Assurance Each lot is tested for: • Lymphocyte separation and recovery. • Lymphocyte viability. • Sterility. • Consistent density (1.077-1.080 at 20°C). Packaging • Packaged in amber, screw-cap bottles. • 5 x 100 ml bottles per carton. Storage. • Stored at room temperature. Reference Boyum, A. (1968): Isolation of mononuclear cells and granulocytes from human blood. Scand J. Clin. Lab. Invest. 21, Suppl. 97. Aspirate I IC[OI I IC~ LJI Catalog number: 8410-01 & discard serum Lymphocyte For Laboratory Use Aspirate layer & use (mononuclear Please write for our current Price List and Catalog. cells and Original platelets) ITi BIONETICS° LSM layer Erythrocytes Laboratory Products and Litton granulocytes 5516 Nicholson Lane, Kensington, Maryland 20795 Telephone: (301) 881-1557 1979 Litton Bionetics, tnc Get the most out of your high quality cytotoxic antibodies with LOW-TOX-M RABBIT COMPLEMENT LOW TOXICITY HIGH ACTIVITY Presentation: CL 3051 5 x 1 ml, lyophilized $30.00 When it comes to COMPLEMENT... come to CEDARLANE Direct orders or inquiries to: UNITED STATES: WORLDWIDE EXCEPT U.S. ,4 C~L CEDARLANE ACCURATE CHEMICAL & LABORATO RI ES SCIENTIFIC CORPORATION LIMITED 5516-8TH LINE, R.R. 2 28 TEC STREET, HICKSVtLLE, N.Y. 11801 HORNBY, ONTARIO, CANADA LOP 1E0 Telephone -

Induction of Protein Citrullination and Auto-Antibodies Production In
www.nature.com/scientificreports OPEN Induction of protein citrullination and auto-antibodies production in murine exposed to nickel Received: 11 November 2015 Accepted: 21 December 2017 nanomaterials Published: xx xx xxxx Bashir M. Mohamed1,7, Noreen T. Boyle2,3, Anja Schinwald4, Bruno Murer5, Ronan Ward6, Omar K. Mahfoud1, Tatsiana Rakovich1, Kieran Crosbie-Staunton1, Steven G. Gray 7, Ken Donaldson4, Yuri Volkov1,2 & Adriele Prina-Mello 1,2 Citrullination, or the post-translational deimination of polypeptide-bound arginine, is involved in several pathological processes in the body, including autoimmunity and tumorigenesis. Recent studies have shown that nanomaterials can trigger protein citrullination, which might constitute a common pathogenic link to disease development. Here we demonstrated auto-antibody production in serum of nanomaterials-treated mice. Citrullination-associated phenomena and PAD levels were found to be elevated in nanomaterials -treated cell lines as well as in the spleen, kidneys and lymph nodes of mice, suggesting a systemic response to nanomaterials injection, and validated in human pleural and pericardial malignant mesothelioma (MM) samples. The observed systemic responses in mice exposed to nanomaterials support the evidence linking exposure to environmental factors with the development of autoimmunity responses and reinforces the need for comprehensive safety screening of nanomaterials. Furthermore, these nanomaterials induce pathological processes that mimic those observed in Pleural MM, and therefore require further investigations into their carcinogenicity. Citrullination is involved in several pathological processes in the body, including autoimmunity and tumor- igenesis. Citrullinated proteins are generated by a post-translational deimination or demethylimination of polypeptide-bound arginine by a family of Ca2+-dependent enzyme peptidylarginine deiminase (PAD)1. -

Antifungal Effects of Lactobacillus Species Isolated from Local Dairy Products
[Downloaded free from http://www.jpionline.org on Sunday, September 17, 2017, IP: 78.39.35.67] Original Research Article Antifungal effects of Lactobacillus species isolated from local dairy products Sahar Karami, Mohammad Roayaei, Elnaz Zahedi, Mahmoud Bahmani1, Leila Mahmoodnia2, Hosna Hamzavi, Mahmoud Rafieian-Kopaei3 Department of Biology, Faculty of Science, Shahid Chamran University of Ahvaz, Ahwaz, 1Department of Pharmacology, Biotechnology and Medicinal Plants Research Center, Ilam University of Medical Sciences, Ilam, 3Department of Pharmacology, Medical Plants Research Center, Basic Health Sciences Institute, 2Department of Internal Medicine, Shahrekord University of Medical Sciences, Shahrekord, Iran Abstract Objective: The Lactobacillus is a genus of lactic acid bacteria which are regularly rod‑shaped, nonspore, Gram‑positive, heterogeneous, and are found in a wide range of inhabitants such as dairy products, plants, and gastrointestinal tract. A variety of antimicrobial compounds and molecules such as bacteriocin are produced by these useful bacteria to inhibit the growth of pathogenic microbes in the food products. This paper aims to examine the isolation of Lactobacillus from local dairies as well as to determine their inhibition effect against a number of pathogens, such as two fungi: Penicillium notatum and Aspergillus fulvous. Materials and Methods: Twelve Lactobacillus isolates from several local dairies. After initial dilution (10−1–10−3) and culture on the setting, de Man, Rogosa and Sharpe‑agar, the isolates were recognized and separated by phenotypic characteristics and biochemical; then their antifungal effect was examined by two methods. Results: Having separated eight Lactobacillus isolates, about 70% of the isolates have shown the inhabiting areas of antifungus on the agar‑based setting, but two species Lactobacillus alimentarius and Lactobacillus delbrueckii have indicated a significant antifungal effect against P. -

Heritability of Autoantibody Levels in a Twin Population
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2009 Heritability of Autoantibody Levels in a Twin Population Amal Rastogi Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Periodontics and Periodontology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/1854 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. School of Dentistry Virginia Commonwealth University This is to certify that the thesis prepared by Amal Rastogi, DMD, PhD entitled HERITABILITY OF AUTOANTIBODY LEVELS IN A TWIN POPULATION has been approved by his committee as satisfactory completion of the thesis requirement for the degree of Master of Science in Dentistry. John Gunsolley, DDS, MS, Professor, Department of Periodontics, VCU, School of Dentistry Harvey Schenkein, DDS, PhD, Chair, Department of Periodontics, VCU, School of Dentistry Robert Sabatini, DDS, MS, Assistant Professor, Department of Periodontics, VCU, School of Dentistry Thomas Waldrop, DDS, MS, Graduate Director, Department of Periodontics, VCU, School of Dentistry Harvey Schenkein, DDS, PhD, Chair, Department of Periodontics, VCU, School of Dentistry Laurie Carter, DDS, PhD, Director of Advanced Dental Education,VCU, School of Dentistry Dr. F. Douglas Boudinot, Dean of the Graduate School, VCU June 29, 2009 © Amal Rastogi, DMD, PhD 2009 All Rights Reserved 2 HERITABILITY OF AUTOANTIBODY LEVELS IN A TWIN POPULATION A thesis submitted in partial fulfillment of the requirements for the degree of MSD at Virginia Commonwealth University. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

Antibody (ANCA)-Associated Vasculitis Avacopan Introduction
CO-1 Avacopan for the Treatment of Anti-Neutrophil Cytoplasmic Auto- antibody (ANCA)-Associated Vasculitis ChemoCentryx, Inc. Arthritis Advisory Committee May 6, 2021 CO-2 Avacopan Introduction Thomas J. Schall, Ph.D. President, Chief Executive Officer ChemoCentryx, Inc. CO-3 Avacopan: First-in-Class, Targeted Therapy for ANCA-Associated Vasculitis ANCA-associated vasculitis is rare, severe, and often fatal autoimmune disease Anti-neutrophil cytoplasmic auto-antibodies (ANCA) involved in pathogenesis Inflammation of small vessels, can affect any organ Commonly affects kidneys Glucocorticoid treatment associated with significant toxicities Despite current therapies, > 1 in 10 patients die within first year of diagnosis1,2 1. Heijl et al., 2017; 2. Little et al., 2010 CO-4 Central Role of C5a in Pathogenesis of ANCA-Associated Vasculitis Jennette and Falk, 2014 CO-5 Avacopan: Highly Potent and Selective C5aR Inhibitor Avacopan avoids long-term 1 biological consequences of ‘upstream’ complement inhibition 1 Does not block C5b-9 production; leaves host defense mechanism C5aC5a AntibodiesAntibodies 2 membrane attack complex (MAC) in place C6-C9C6-C9 AvacopanAvacopan Preserves beneficial 3 functions of C5L2 pathway C5aR Leukocyte migration and signaling Leukocyte trafficking, Cell lysis migration and (i.e., Neisseria control activation Beneficial Targets ‘downstream' anti-inflammatoryanti-inflammatory 4 effect complement pathway Adaptive Phagocytosis 4 3 2 signaling and clearance CO-6 Avacopan in ANCA-Associated Vasculitis Pirow Bekker, MD, PhD Clinical Lead Avacopan Clinical Development Program ChemoCentryx, Inc. CO-7 Avacopan Proposed Indication and Dose Proposed Indication …for the treatment of adult patients with anti-neutrophil cytoplasmic auto-antibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis and microscopic polyangiitis). -

Degradation of Neutrophil Extracellular Traps Is Decreased in Patients with Antiphospholipid Syndrome J
Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome J. Leffler1, L. Stojanovich2, Y. Shoenfeld3, G. Bogdanovic2, R. Hesselstrand4, A.M. Blom1 1Dept. of Laboratory Medicine, Section of Medical Protein Chemistry, Lund University, Malmö, Sweden; 2Dept. of Internal Medicine, “Bezhanijska Kosa” University Medical Center, Belgrade, Serbia; 3Zabludowicz Center for Autoimmune Diseases, Sheba Medical Center, Tel Aviv, Israel; 4Dept. of Clinical Sciences, Section of Rheumatology, Lund University, Lund, Sweden. Abstract Objective A decreased ability to degrade neutrophil extracellular traps (NETs) is seen in a subgroup of patients with systemic lupus erythematosus (SLE) and correlates with the presence of autoantibodies. Antiphospholipid syndrome (APS) can develop secondary to SLE or as a primary disease. In the current study we investigated the ability of sera from patients with APS to degrade NETs. The presence of antibodies against NETs and neutrophil remnants were also determined in the same patients. Methods In the study, 106 patients with APS (73 primary and 33 secondary), 76 patients with systemic sclerosis (SSc) and 77 healthy donors as control samples were included. NETs generated from neutrophils isolated from healthy volunteers were incubated with patient sera, followed by measurement of degraded NETs or deposited IgG. Results Sera of APS patients had a decreased ability to degrade NETs compared to healthy controls, with no difference between primary and secondary APS. Sera from SSc patients did not differ significantly from healthy controls in the ability to degrade NETs. A decreased degradation of NETs correlated weakly to increased levels of antibodies against NETs/ neutrophil remnants in patients with primary APS, but stronger in patients with secondary APS.