Developmental Biology 448 (2019) 247–259
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Appendicularia of CTAW
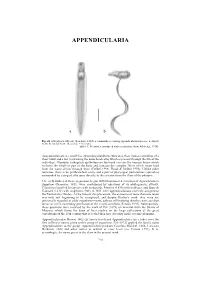
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Redalyc.Checklist Da Classe Appendicularia (Chordata: Tunicata)
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Vega-Pérez, Luz Amelia; Gurgel de Campos, Meiri Aparecida; Schinke, Katya Patrícia Checklist da Classe Appendicularia (Chordata: Tunicata) do Estado de São Paulo, Brasil Biota Neotropica, vol. 11, núm. 1a, 2011, pp. 1-9 Instituto Virtual da Biodiversidade Campinas, Brasil Disponível em: http://www.redalyc.org/articulo.oa?id=199120113029 Como citar este artigo Número completo Sistema de Informação Científica Mais artigos Rede de Revistas Científicas da América Latina, Caribe , Espanha e Portugal Home da revista no Redalyc Projeto acadêmico sem fins lucrativos desenvolvido no âmbito da iniciativa Acesso Aberto Checklist da classe appendicularia (Chordata: Tunicata) do Estado de São Paulo, Brasil Vega-Pérez, L.M. et al. Biota Neotrop. 2011, 11(1a): 000-000. On line version of this paper is available from: http://www.biotaneotropica.org.br/v11n1a/en/abstract?inventory+bn0401101a2011 A versão on-line completa deste artigo está disponível em: http://www.biotaneotropica.org.br/v11n1a/pt/abstract?inventory+bn0401101a2011 Received/ Recebido em 23/07/2010 - Revised/ Versão reformulada recebida em 08/10/2010 - Accepted/ Publicado em 15/12/2010 ISSN 1676-0603 (on-line) Biota Neotropica is an electronic, peer-reviewed journal edited by the Program BIOTA/FAPESP: The Virtual Institute of Biodiversity. This journal’s aim is to disseminate the results of original research work, associated or not to the program, concerned with characterization, conservation and sustainable use of biodiversity within the Neotropical region. Biota Neotropica é uma revista do Programa BIOTA/FAPESP - O Instituto Virtual da Biodiversidade, que publica resultados de pesquisa original, vinculada ou não ao programa, que abordem a temática caracterização, conservação e uso sustentável da biodiversidade na região Neotropical. -

Distribution and Abundance of Larvaceans in the Southern Ocean
Distribution and abundance of Larvaceans in the Southern Ocean By Margaret Caroline Murray LINDSAY BSc. (Env. Mang. & Eco) Hons. GCAS Submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy University of Tasmania June 2012 _________________________ _______ _____ _ Declaration of originality______________________ This thesis contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright. Margaret CM Lindsay June 2012 ___ ____________________________ ______ _____ Statement of authority of access___ _____________ This thesis may be made available for loan and limited copying in accordance with the Copyright Act 1968. Margaret CM Lindsay June 2012 _______________________________ _ ________ _ Statement regarding published work contained in the thesis___ __ _______ ___ The publishers of the papers comprising Chapter 5 hold the copyright for that content, and access to the material should be sought from the respective journals. The remaining non published content of the thesis may be available for loan and limited copying in accordance with the Copyright Act 1968. Margaret CM Lindsay June 2012 ii _______________________________ _ _________ Statement of co-authorship____ ___ ___________ The following people and institutions contributed to the publication of the work undertaken as part of this thesis: Chapter 5: Distribution and abundance of Larvaceans in the Southern Ocean between 30° and 80°E. -

Species-Specific House Productivity of Appendicularians
MARINE ECOLOGY PROGRESS SERIES Vol. 259: 163–172, 2003 Published September 12 Mar Ecol Prog Ser Species-specific house productivity of appendicularians Riki Sato1, 2,*, Yuji Tanaka1, Takashi Ishimaru1 1Department of Ocean Sciences, Tokyo University of Fisheries, 4-5-7 Konan, Minato-ku, Tokyo 108-8477, Japan 2Present address: Louisiana Universities Marine Consortium, 8124 Highway 56, Chauvin, Louisiana 70344, USA ABSTRACT: Appendicularians, pelagic tunicates that are common in world oceans, periodically pro- duce new mucus houses and discard old ones. Discarded houses form macroscopic aggregates that constitute sites of biological activity in the water column and also contribute to the transport of organic matter to deeper water. In this study, we measured the house renewal rates of 10 appendic- ularian species cultivated in 30 µm-mesh-screened seawater at 17 to 29°C. In addition, the carbon content of the tunicates (CB), their newly secreted houses (CNH) and their discarded houses (CDH) were concurrently examined for some of the oikopleurid species. House renewal rates varied from 2 houses d–1 for Oikopleura cophocerca at 20°C to 40 houses d–1 for Fritillaria formica digitata at 23°C. The CNH of O. longicauda, O. fusiformis, O. rufescens and Megalocercus huxleyi were 0.16, 0.48, 1.6 and 8.8 µg, corresponding to 5.3, 9.2, 14.1 and 10.3% of CB, respectively; the CDH of the first 3 species were 0.68, 1.2 and 3.9 µg, corresponding to 17.9, 30.0 and 32.8% of CB, respectively (CDH was not measured in M. huxleyi). -

Dinâmica Populacional De Appendicularia E Cladocera Na Plataforma Interna De Ubatuba (SP): Um Estudo Sazonal E Multianual
Leonardo Kenji Miyashita Dinâmica populacional de Appendicularia e Cladocera na plataforma interna de Ubatuba (SP): um estudo sazonal e multianual Dissertação apresentada ao Instituto Oceanográfico da Universidade de São Paulo, como parte dos requisitos para a obtenção do Título de Mestre em Ciências, área de Oceanografia Biológica. Orientador: Prof. Dr. Rubens Mendes Lopes São Paulo 2010 Universidade de São Paulo Instituto Oceanográfico Dinâmica populacional de Appendicularia e Cladocera na plataforma interna de Ubatuba (SP): um estudo sazonal e multianual Leonardo Kenji Miyashita Dissertação apresentada ao Instituto Oceanográfico da Universidade de São Paulo, como parte dos requisitos para obtenção do título de Mestre em Ciências, área de Oceanografia Biológica. Julgada em ____/____/____ por _____________________________________ _______________ Prof(a). Dr(a). Conceito _____________________________________ _______________ Prof(a). Dr(a). Conceito _____________________________________ _______________ Prof(a). Dr(a). Conceito i Agradecimentos Primeiramente gostaria de agradecer a Fapesp (processo nº 2007/56931-1) pela bolsa concedida, sem a qual não seria possível a realização deste trabalho. A bolsa também possibilitou minha participação no VIII International Symposium on Cladocera, uma experiência bastante positiva, pois tive a oportunidade de apresentar uma parte deste trabalho, de assistir ótimas palestras e de conhecer pesquisadores renomados. Sou extremamente grato ao meu orientador, prof. Dr. Rubens M. Lopes, pela excelente orientação e todos os ensinamentos passados, além de me proporcionar um ótimo ambiente de trabalho, onde sempre tive tudo que precisei para realizar minha pesquisa com tranquilidade. Ao prof. Dr. Salvador A. Gaeta por possibilitar a participação no projeto Antares, pela disponibilização dos dados de clorofila a e pela realização dos “jantares”. -

Title STUDIES on the DISTRIBUTION OF
STUDIES ON THE DISTRIBUTION OF APPENDICULARIANS AND SOME THALIACEANS OF Title THE NORTH PACIFIC, WITH SOME MORPHOLOGICAL NOTES Author(s) Tokioka, Takasi PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1960), 8(2): 351-443 Issue Date 1960-12-20 URL http://hdl.handle.net/2433/174644 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University STUDIES ON THE DISTRIBUTION OF APPENDICULARIANS AND SOME THALIACEANS OF THE NORTH PACIFIC, WITH SOME MORPHOLOGICAL NOTES'J TAKAS! TOKIOKA Seto Marine Biological Laboratory, Siraharna With 16 Text-figures and 36 Tables CONTENTS Page INTRODUCTION .......................................................................................... 352 MATERIAL AND METHODS ........................................................................ 353 APPENDICULARIANS OCCURRED IN THE WHOLE COLLECTIONS TREATED IN THIS PAPER ................................................................................. 354 OCCURRENCE OF APPENDICULARIANS AND SOME OTHER PELAGIC TUNI- CATES IN RESPECTIVE COLLECTIONS ................................................... 356 I. Appendicularians found in the samples collected in the subarctic waters .......................................................................................... 356 II. Appendicularians in the mixing area between the subarctic water and the warm water .. 00 00 00 00 00 00 00 00 .. 00 00 00 00 00 00 00 00 00 00 00 00 00 00 00 oo. oo oo ............ 356 III. Pelagic tunicates in the blue-green water along the southern Cali- fornian -

Universidad De Costa Rica Facultad De Ciencias Escuela De Biología Tesis Presentada Para Optar Al Grado De Licenciatura En Biol
Universidad de Costa Rica Facultad de Ciencias Escuela de Biología Tesis presentada para optar al grado de Licenciatura en Biología con énfasis en Zoología Taxonomía y distribución geográfica de los tunicados pelágicos en Costa Rica Marco Corrales Ugalde Ciudad Universitaria Rodrigo Facio 2014 i MIEMBROS DEL TRIBUNAL _______________________________ ___________________________ Dr.rer.nat. Álvaro Morales Ramírez Ph.D. Gustavo Gutierrez Espeleta Director de tesis Decano Facultad Ciencias Básicas ________________________________ ____________________________ Dr. Jorge Cortés Núñez Dr. Jeffrey Sibaja Cordero Miembro del Tribunal Miembro del Tribunal ________________________________ ________________________________ M. Sc. Monika Springer Marco Vinicio Corrales Ugalde Revisor externo Candidato ii A mi padre, que me enseño el verdadero valor del trabajo, a mi madre, que me demostró el poder del conocimiento, a mi hermana, que me hizo entender la fuerza de la inocencia y a Karla, quien me tomó de la mano y se atrevió a soñar conmigo. iii “Gozas, hundiendo el cuerpo en el vivo oleaje; lo acarician tus ojos y tus brazos; tu oído se distrae muchas veces de tu propio gemido, al rumor de su canto indomable y salvaje.” Charles Baudelaire El Mar Si arruga el hosco ceño desata tempestades, que forman y deshacen líquidos ventisqueros; la espuma desflecada que adorna sus cimeros vestía en otro tiempo sirenas y deidades. Desde que se miraron en remotas edades la noche y él han sido camaradas sinceros: por eso ella le brinda su copa de luceros para enjoyar el manto de sus sinuosidades. Hoy que su indócil lomo domeña el trasatlántico: su rumor no tiene sonoridad de cántico. Afrodita no existe. Neptuno es solo un nombre. -

Archiv Für Naturgeschichte
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Archiv für Naturgeschichte Jahr/Year: 1895 Band/Volume: 60-2_3 Autor(en)/Author(s): Matzdorff Carl Artikel/Article: Jahresbericht über die Tunicaten für 1894, 1895 und 1896. 7-64 — © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Jahresbericht über die Tunicaten für 1894, 1895 und 1896, Von Dr. Carl Matzdorff, Oberlehrer in Pankow bei Berlin. A. Allgemeines und Vermischtes. 1. Cultur. Lendenfeld, R. yon. Ueber meinen Aqnarienfilter (Zool. Anz., 19. B., Leipzig, 1896, S. 95). In häufig durch Knochenkohle filtrirtem Seewasser halten sich Ascidien jahrelang. 2, Conservirutiff, Tullberg, T. Ueber Conservirung von Evertebraten in aus- gedehntem Zustande (*Biol. Foren. Förh., IV, S. 4 — 9.). Auszug von Racovitza: Sur la conservation des invertebres ä l'etat d'epanou- issement (Archiv. Zool. exper., 2. serie, t. 10, Paris, 1893, S.XI XIV). Vgl. auch: Natwiss. Rundsch., 7. B., S. 294. Das Verfahren, das auch an Ciona intestinalis erprobt wurde, besteht in einer Anästhetisirung durch Magnesiumchlorid oder -Sulfat (1 : 100) und darauf folgendem langsamen Abtöten durch Chromsäm-e. Redenbaugh, W. A. Preservation of some Marine Animals. (Amer. Natur., V. 29, Philadelphia, 1895, p. 399—401.) Molgula und Cynthia wurden mit offenen Siphonen getötet, nachdem sie mit Magnesiumsulfat anästhetisirt waren. Die ge- sättigte Sulfatlösung wurde mit der Pipette eingebracht. Hinzu- fügung von 0,P/o Chromsäurelösung bis zur Beschickung des Wassers mit 0,03 bis 0,05% Säure gab auch gute Erfolge, doch schrumpfte der Umfang etwas ein. Collin, A. Mantelthiere (Tunikaten). In: Anleitung zum Sammeln, Konserviren und Verpacken von Thieren für die zoologische 8 © Biodiversity HeritageDr.