Usher Syndrome the Primary Content of This Article Was Retrieved From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
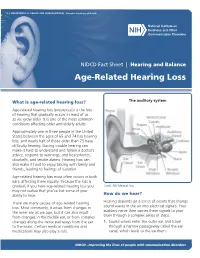
Age-Related Hearing Loss
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Age-Related Hearing Loss What is age-related hearing loss? The auditory system Age-related hearing loss (presbycusis) is the loss of hearing that gradually occurs in most of us as we grow older. It is one of the most common conditions affecting older and elderly adults. Approximately one in three people in the United States between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing. Having trouble hearing can make it hard to understand and follow a doctor’s advice, respond to warnings, and hear phones, doorbells, and smoke alarms. Hearing loss can also make it hard to enjoy talking with family and friends, leading to feelings of isolation. Age-related hearing loss most often occurs in both ears, affecting them equally. Because the loss is gradual, if you have age-related hearing loss you Credit: NIH Medical Arts may not realize that you’ve lost some of your ability to hear. How do we hear? There are many causes of age-related hearing Hearing depends on a series of events that change loss. Most commonly, it arises from changes in sound waves in the air into electrical signals. Your the inner ear as we age, but it can also result auditory nerve then carries these signals to your from changes in the middle ear, or from complex brain through a complex series of steps. changes along the nerve pathways from the ear 1. -

Novel Association of Hypertrophic Cardiomyopathy, Sensorineural Deafness, and a Mutation in Unconventional Myosin VI (MYO6)
309 LETTER TO JMG J Med Genet: first published as 10.1136/jmg.2003.011973 on 1 April 2004. Downloaded from Novel association of hypertrophic cardiomyopathy, sensorineural deafness, and a mutation in unconventional myosin VI (MYO6) S A Mohiddin, Z M Ahmed, A J Griffith, D Tripodi, T B Friedman, L Fananapazir, R J Morell ............................................................................................................................... J Med Genet 2004;41:309–314. doi: 10.1136/jmg.2003.011973 amilial hypertrophic cardiomyopathy (FHC) is typically Key points characterised by left ventricular hypertrophy, diastolic Fdysfunction, and hypercontractility, and is often asso- ciated with disabling symptoms, arrhythmias, and sudden N Familial hypertrophic cardiomyopathy (FHC) is typi- death.1 FHC shows both non-allelic and allelic genetic cally confined to a cardiac phenotype and is caused by heterogeneity, and results from any one of more than 100 mutations in genes encoding sarcomeric proteins. mutations in genes encoding sarcomeric proteins.2 Identified Occasionally FHC may be one component of a genes include those encoding b myosin heavy chain, the hereditary multisystem disorder. myosin regulatory and essential light chains, myosin bind- N Sensorineural hearing loss is genetically heteroge- ing protein C, troponin I, troponin C, a cardiac actin, and neous. Mutations in the MYO6 gene, encoding 23 titin. The FHC phenotype is characterised by hypertrophy, unconventional myosin VI, have been found to cause myocyte disarray and fibrosis, and results from the dominant non-syndromic sensorineural hearing loss—that is, negative expression of one of these (mainly missense) sensorineural hearing loss in the absence of any other mutations. The resulting sarcomeric dysfunction leads related clinical features. ultimately, through mechanisms that remain obscure, to pathological left ventricular remodelling. -

Visual Field Test
EYE FACTS visual field test Your visual field refers to how much you can see around you, including objects in your peripheral (side) vision. This test produces a map of your field of vision. Visual field tests help your ophthalmologist (Eye M.D.) monitor any loss of vision and diagnose eye problems and disease. Visual field testing is used to monitor peripheral, or side, vision. HOW IS A VISUAL FIELD TEST PERFORMED? The test is performed with a large, bowl-shaped in- Normal visual field Severe visual loss strument called a perimeter. In order to test one eye at a time, one of your eyes is temporarily patched during the test. You will be seated and positioned comfortably in front of the perimeter and asked to look straight ahead at a fixed spot (the fixation target). The computer randomly flashes points of light around the bowl-shaped perimeter. When you see a light, press the indicator button. It is very important These grids are results of visual field tests.T he dark to always keep looking straight ahead. Do not move black shaded areas show where loss of vision has your eyes to look for the target; wait until it appears occurred. in your side vision. It is normal for some of the lights to be difficult to see. A delay in seeing a light does not necessarily mean your field of vision is damaged. If you need to rest during the test, tell the technician and they will pause the test until you are ready to continue. Your ophthalmologist will interpret the results of your test and discuss them with you. -

Optic Disc and Macular Vessel Density Measured by Optical
www.nature.com/scientificreports OPEN Optic Disc and Macular Vessel Density Measured by Optical Coherence Tomography Angiography in Open-Angle and Angle-Closure Glaucoma Tzu-Yu Hou1,2, Tung-Mei Kuang1,2, Yu-Chieh Ko1,2, Yu-Fan Chang1,2, Catherine Jui-Ling Liu1,2 & Mei-Ju Chen1,2* There is distinct pathogenesis between primary open-angle glaucoma (POAG) and primary angle- closure glaucoma (PACG). Although elevated intraocular pressure (IOP) is the major risk factor for glaucoma, non-IOP risk factors such as vascular abnormalities and lower systolic/diastolic perfusion pressure may play a role in the pathogenic process. This study aimed to compare the vessel density (VD) in the optic disc and macula using optical coherence tomography angiography (OCTA) between POAG and PACG eyes. Thirty-two POAG eyes, 30 PACG eyes, and 39 control eyes were included. All the optic disc VD parameters except the inside disc VD were signifcantly lower in glaucomatous eyes than in control eyes. Compared with PACG eyes, only the inferior temporal peripapillary VD was signifcantly lower in POAG eyes. The parafoveal VD was signifcantly lower in each quadrant in glaucomatous eyes than in control eyes. The central macular and parafoveal VD did not difer between POAG and PACG eyes. In conclusion, the inferior temporal peripapillary VD was signifcantly reduced in POAG eyes compared with PACG eyes, while PACG eyes showed a more evenly distributed reduction in the peripapillary VD. The distinct patterns of VD change may be associated with the diferent pathogenesis between POAG and PACG. Glaucoma is an optic neuropathy characterised by progressive loss of retinal ganglion cells and their axons accompanied by corresponding visual feld (VF) defects. -

Physical Eye Examination
Physical Eye Examination Kaevalin Lekhanont, MD Department of Ophthalmology Ramathibodi Hospp,ital, Mahidol Universit y Outline • Visual acuity (VA) testing – Distant VA test – Pinhole test – Near VA test • Visual field testing • Record and interpretations Outline • Penlight examination •Swingggping penli ght test • Direct ophthalmoscopy – Red reflex examination • Schiotz tonometry • RdditttiRecord and interpretations Conjunctiva, Sclera Retina Cornea Iris Retinal blood vessels Fovea Pupil AtAnteri or c ham ber Vitreous Aqueous humor Lens Optic nerve Trabecular meshwork Ciliary body Choriod and RPE Function evaluation • Visual function – Visual acuity test – Visual field test – Refraction • Motility function Anatomical evaluation Visual acuity test • Distant VA test • Near VA test Distance VA test Snellen’s chart • 20 ฟุตหรือ 6 เมตร • วัดที่ละขาง ตาขวากอนตาซาย • ออานทละตาานทีละตา แถวบนลงลแถวบนลงลางาง • บันทึกแถวลางสุดที่อานได Pinhole test VA with pinhole (PH) Refractive error emmetitropia myypopia hyperopia VA record 20/200 ผูปวยสามารถอานต ัวเลขทมี่ ี ขนาดใหญขนาดใหญพอทคนปกตพอที่คนปกติ สามารถอานไดจากท ี่ระยะ 200 ฟตฟุต แตแตผผปูปวยอานไดจากวยอานไดจาก ที่ระยะ 20 ฟุต 20/20 Distance VA test • ถาอานแถวบนสุดไไไมได ใหเดินเขาใกล chthart ทีละกาวจนอานได (10/200, 5/200) • Counting finger 2ft - 1ft - 1/2ft • Hand motion • Light projection • Light perception • No light perception (NLP) ETDRS Chart Most accurate Illiterate E chart For children age ≥ 3.5 year Near VA test Near chart •14 นวิ้ หรอื 33 เซนตเมตริ • วัดที่ละขาง ตาขวากอนตาซาย • อานทีละตา แถวบนลงลาง -

Clinical Genetics and the Hutterite Brethren
Clinical Genetics and the Hutterite Brethren: What have we learned in the new millenium? Or: A Micheil Innes MD FRCPC FCCMG Adapted from: Medical Genetics Grand Rounds January 2013 History and Population Hutterite Population Today >40 000 in AB, 30000 MB, ND, SD 1593-1770 1874-1879 25000 Transylvania 1256 migrated to American Prairies 20000 15000 World War I 10000 1565-1592 1770 - 1870 Migration to Canada Moravia5000 Ukraine 0 1500s 1520 1540 1550 1570 1580 1590 1610 1620 1680 1750 1760 1840 1860 1890 1900 1950 1975 1990 Tyrolean Alps Why Identify Genes in this Population? • Direct Benefits to • Benefit to Larger Patients/Families population – Non-invasive – Most of these disorders diagnostic test are not confined to this – Carrier test (*marriage population restrictions) – May allow for diagnosis – ?Prenatal testing of atypical cases – Enhanced understanding of – Expand basic science disease may facilitate and clinical knowledge management or treatment Initial Presentations May be Non-Specific Highlighting Importance of Careful Syndrome Delineation and Early Genetic Diagnosis • Hearing Loss – Autosomal recessive non-syndromic hearing loss (> 2loci) – Usher syndrome (> 2loci) – HDR syndrome • Cerebellar Ataxia – Joubert syndrome – DES syndrome – DCMA syndrome – CASS syndrome • Muscular Dystrophy/ High CK – LGMD2H – LGDM2I – AR EDMD – Myopathy with CPEO – Microcephaly with Chorea Genetic services and the Hutterites Religion/Culture • Has posed little barrier overall • Very accepting of medical care and technology • Although they believe that God plays a day to day role in guiding their lives, most couples accept genetic explanations for their children’s disorders • Some leuts and individual colonies are more conservative than others • Colony leader is clearly the Minister • Who speaks for the overall community when it comes to community wide issues? – e.g. -

Splicing-Correcting Therapeutic Approaches for Retinal Dystrophies: Where Endogenous Gene Regulation and Specificity Matter
New Developments Splicing-Correcting Therapeutic Approaches for Retinal Dystrophies: Where Endogenous Gene Regulation and Specificity Matter Niccolo` Bacchi,1 Simona Casarosa,1,2 and Michela A. Denti1,3 1Centre for Integrative Biology (CIBIO) - University of Trento, Trento, Italy 2Neuroscience Institute - National Research Council (CNR), Pisa, Italy 3Neuroscience Institute - National Research Council (CNR), Padova, Italy Correspondence: Simona Casarosa, Splicing is an important and highly regulated step in gene expression. The ability to modulate Centre for Integrative Biology it can offer a therapeutic option for many genetic disorders. Antisense-mediated splicing- (CIBIO) - University of Trento, Via correction approaches have recently been successfully exploited for some genetic diseases, Sommarive 9, 38123 Trento, Italy; and are currently demonstrating safety and efficacy in different clinical trials. Their [email protected]. application for the treatment of retinal dystrophies could potentially solve a vast panel of Michela A. Denti, Centre for Inte- grative Biology (CIBIO) - University cases, as illustrated by the abundance of mutations that could be targeted and the versatility of ofTrento,ViaSommarive9,38123 the technique. In this review, we will give an insight of the different therapeutic strategies, Trento, Italy; focusing on the current status of their application for retinal dystrophies. [email protected]. Keywords: splicing correction, antisense oligonucleotides, retinal dystrophy, gene therapy SC and MAD contributed equally to the work presented here and should therefore be regarded as equivalent authors. Submitted: April 8, 2014 Accepted: April 11, 2014 Citation: Bacchi N, Casarosa S, Denti MA. Splicing-correcting therapeutic approaches for retinal dystrophies: where endogenous gene regulation and specificity matter. Invest Oph- thalmol Vis Sci. -
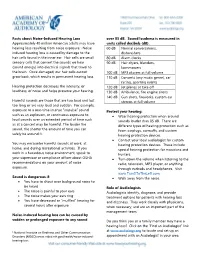
Facts About Noise-Induced Hearing Loss Over 85 Db
Facts about Noise-Induced Hearing Loss over 85 dB. Sound loudness is measured in Approximately 40 million American adults may have units called decibels (dB). hearing loss resulting from noise exposure.1 Noise- 60 dB Normal conversations, induced hearing loss is caused by damage to the dishwashers hair cells found in the inner ear. Hair cells are small 80 dB Alarm clocks sensory cells that convert the sounds we hear 90 dB Hair dryers, blenders, (sound energy) into electrical signals that travel to lawnmowers the brain. Once damaged, our hair cells cannot 100 dB MP3 players at full volume grow back, which results in permanent hearing loss. 110 dB Concerts (any music genre), car racing, sporting events Hearing protection decreases the intensity, or 120 dB Jet planes at take off loudness, of noise and helps preserve your hearing. 130 dB Ambulance, fire engine sirens 140 dB Gun shots, fireworks, custom car Harmful sounds are those that are too loud and last stereos at full volume too long or are very loud and sudden. For example, exposure to a one-time intense “impulse” sound Protect your hearing: such as an explosion, or continuous exposure to • Wear hearing protection when around loud sounds over an extended period of time such sounds louder than 85 dB. There are as at a concert may be harmful. The louder the different types of hearing protection such as sound, the shorter the amount of time you can foam earplugs, earmuffs, and custom safely be around it. hearing protection devices • Contact your local audiologist for custom You may encounter harmful sounds at work, at hearing protection devices. -

An Investigation of Visual Field Test Parameters in Glaucoma, Patterns Of
An investigation of visual field test parameters in glaucoma, patterns of visual field loss in diabetics and multispectral imaging of the optic nerve head in glaucoma A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy in the Faculty of Medical and Human Sciences 2012 Yanfang Wang School of Medicine (Human Development) 1 CONTENTS Title page……………………………………………………………1 Contents……………………………………………………….........2 List of Tables………………………………………………………..9 List of Figures……………………………………………………..10 List of Abbreviations……………………………………………...14 Abstract …………………………………………………………...16 Declaration………………………………………………………...17 Copyright statement………………………………………………17 Acknowledgment……………………………………………...…..19 1. Rationale of the study…………………………………………..20 2. Glaucoma……………………………………………………….24 2.1- Classification of glaucoma……………………………………….........24 2.2 - Clinical assessment in glaucoma……………………………………..27 2.2.1- IOP measurement………………………………………………..27 2.2.2 - Examination of structural and functional loss in glaucoma….28 2.3 - Management…………………………………………………………..32 3. Visual field testing……………………………………………..33 3.1 - Stimuli and background……………………………………………...33 3.2 - Test strategies………………………………………………………….34 3.2.1 - Frequency-of-seeing (FOS) curve and threshold………………34 3.2.2 - Supra-threshold strategy………………………………………..36 2 3.2.3 - Threshold strategy……………………………………………….38 3.2.3.1 - Full threshold, Fastpac and SITA………………………….38 3.2.3.2 - 30-2, 24-2 and 10-2 stimulus distributions…………………41 3.3 - Interpretation of results……………………………………………...42 3.3.1 -

Bass – Glaucomatous-Type Field Loss Not Due to Glaucoma
Glaucoma on the Brain! Glaucomatous-Type Yes, we see lots of glaucoma Field Loss Not Due to Not every field that looks like glaucoma is due to glaucoma! Glaucoma If you misdiagnose glaucoma, you could miss other sight-threatening and life-threatening Sherry J. Bass, OD, FAAO disorders SUNY College of Optometry New York, NY Types of Glaucomatous Visual Field Defects Paracentral Defects Nasal Step Defects Arcuate and Bjerrum Defects Altitudinal Defects Peripheral Field Constriction to Tunnel Fields 1 Visual Field Defects in Very Early Glaucoma Paracentral loss Early superior/inferior temporal RNFL and rim loss: short axons Arcuate defects above or below the papillomacular bundle Arcuate field loss in the nasal field close to fixation Superotemporal notch Visual Field Defects in Early Glaucoma Nasal step More widespread RNFL loss and rim loss in the inferior or superior temporal rim tissue : longer axons Loss stops abruptly at the horizontal raphae “Step” pattern 2 Visual Field Defects in Moderate Glaucoma Arcuate scotoma- Bjerrum scotoma Focal notches in the inferior and/or superior rim tissue that reach the edge of the disc Denser field defects Follow an arcuate pattern connected to the blind spot 3 Visual Field Defects in Advanced Glaucoma End-Stage Glaucoma Dense Altitudinal Loss Progressive loss of superior or inferior rim tissue Non-Glaucomatous Etiology of End-Stage Glaucoma Paracentral Field Loss Peripheral constriction Hereditary macular Loss of temporal rim tissue diseases Temporal “islands” Stargardt’s macular due -

Visual Field & Otc Tests Care Instructions
DR. CAROLYN ANDERSON EYE SURGERY CARE INSTRUCTIONS VISUAL FIELD & OTC TESTS To ensure the health of your Visual Field Test eye(s), please read this information sheet carefully. Your visual field is the entire area that you can see when the eye is forward, including your peripheral vision. As most of us use two eyes, the overlapping fields allow you to see in an arc of 180 degrees. Certain diseases can cause a loss of visual field, and unless If you need to cancel your the defect is extensive, you will not be aware of it. appointment, please let us know as soon as possible at The Humphrey Field Analyzer 2 uses a computer-controlled, projected beam of light to 604.530.6838. map the visual field, which is then compared by a computer against a database of normal readings. The Visual Field test results are plotted on paper, extending about 90 degrees If you have any questions or to the temple side, and 60 degrees to the nose side. Dr. Anderson will examine the Visual concerns, please speak with Field results, and from the type and location of the defect (if any) can tell where in Dr. Anderson. the visual system the problem may lie. The field test is also used to monitor possible progression of diseases like glaucoma and can indicate if more intensive therapy (if any) is needed. The test is done by a technician and takes approximately 30 minutes. Drops are not generally used, so your vision should not be affected. Appointment date: Time: Optical Coherence Tomographer (OCT) Test The Optical Coherence Tomographer is a type of scanning laser ophthalmoscope, which uses a low-powered laser and a computer to build up a three-dimensional picture of the optic nerve, macula, and other structures in the back of the eye. -

Cardiomyopathy Precision Panel Overview Indications
Cardiomyopathy Precision Panel Overview Cardiomyopathies are a group of conditions with a strong genetic background that structurally hinder the heart to pump out blood to the rest of the body due to weakness in the heart muscles. These diseases affect individuals of all ages and can lead to heart failure and sudden cardiac death. If there is a family history of cardiomyopathy it is strongly recommended to undergo genetic testing to be aware of the family risk, personal risk, and treatment options. Most types of cardiomyopathies are inherited in a dominant manner, which means that one altered copy of the gene is enough for the disease to present in an individual. The symptoms of cardiomyopathy are variable, and these diseases can present in different ways. There are 5 types of cardiomyopathies, the most common being hypertrophic cardiomyopathy: 1. Hypertrophic cardiomyopathy (HCM) 2. Dilated cardiomyopathy (DCM) 3. Restrictive cardiomyopathy (RCM) 4. Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) 5. Isolated Left Ventricular Non-Compaction Cardiomyopathy (LVNC). The Igenomix Cardiomyopathy Precision Panel serves as a diagnostic and tool ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes. Indications The Igenomix Cardiomyopathy Precision Panel is indicated in those cases where there is a clinical suspicion of cardiomyopathy with or without the following manifestations: - Shortness of breath - Fatigue - Arrythmia (abnormal heart rhythm) - Family history of arrhythmia - Abnormal scans - Ventricular tachycardia - Ventricular fibrillation - Chest Pain - Dizziness - Sudden cardiac death in the family 1 Clinical Utility The clinical utility of this panel is: - The genetic and molecular diagnosis for an accurate clinical diagnosis of a patient with personal or family history of cardiomyopathy, channelopathy or sudden cardiac death.