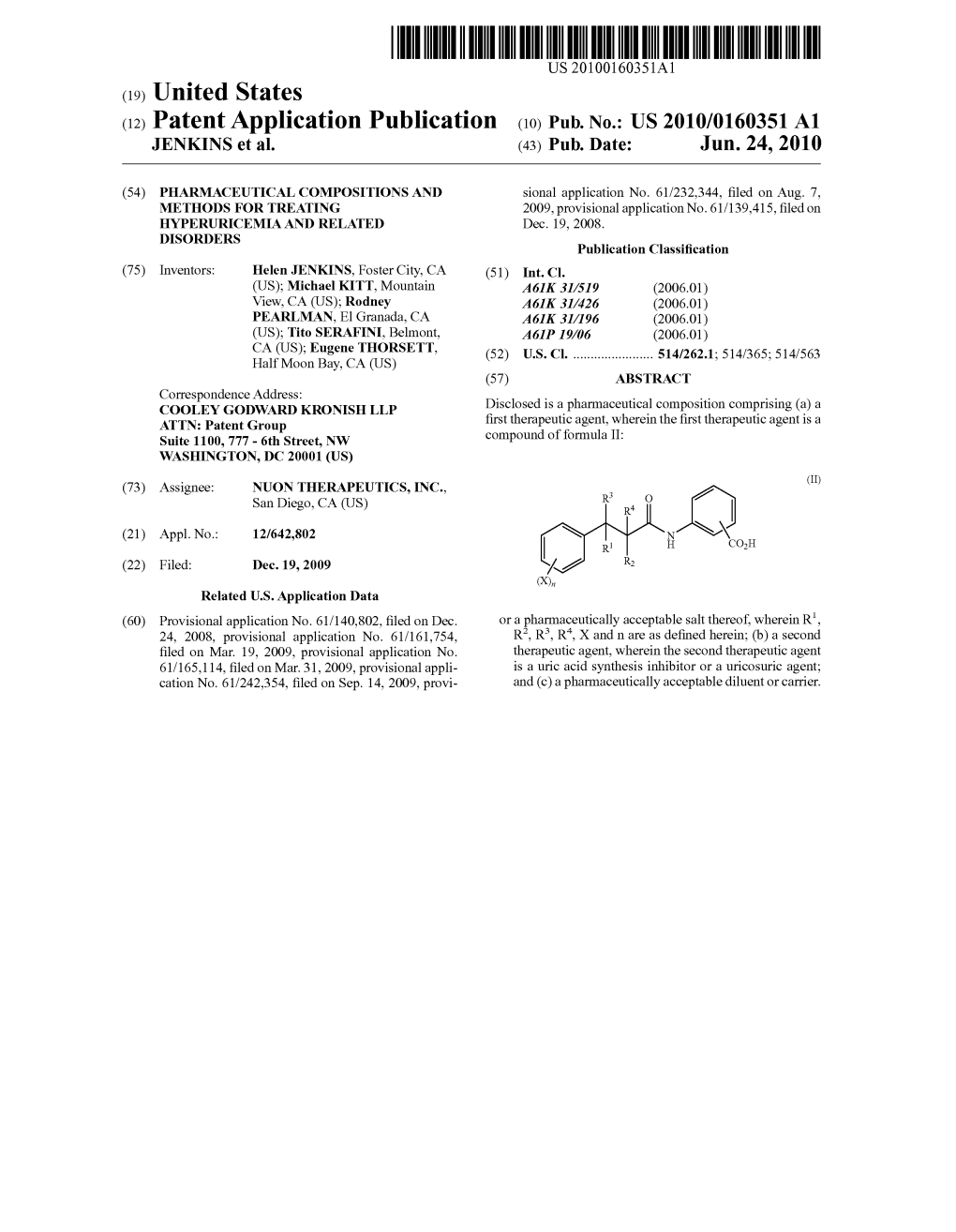
(12) Patent Application Publication (10) Pub. No.: US 2010/0160351A1 UENKNS Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

169 ALLOPUBINOL-INDUCED OROTICACIDURIA 172 in a NEW DUTCH PATIENT with PNP-DEFICIENCY and CELLULAR ITNODEFICIENCY Sebastian Beiter
URINABY OXIPURINOL-1-RIBOSIDE EXCRETION AND RESIDUAL PURINE NUCLEOSIDE PHOSPHORYLASE ACTIVITY 169 ALLOPUBINOL-INDUCED OROTICACIDURIA 172 IN A NEW DUTCH PATIENT WITH PNP-DEFICIENCY AND CELLULAR ITNODEFICIENCY Sebastian Beiter. Werner Loffler, Wolfgang 2 Grobner and Nepomnk Zollner. Medizinische Poliklinik der G.R~ 'ksenl, J.M.Ze ers , lL. J.M. S aa en , W.~uis~,M.Duran , Universitat Miinchen, W-Germany. B.S.:oorbrooc?, G.E.2Staal and S.E.zdman2. 1 Dept. Hematology, Div. Med.Enzymology, University Hospital, Allopurinol-induced oroticaciduria which is due to inhi- Utrecht, The Netherlands. 2 University Children's Hospital "Het bition of OW-decarboxylase by oxipurinol-1- and -7-riboti- Wilhelmina Kinderziekenhuis", Utrecht, The Netherlands. 3 Juli- de is markedly reduced by dietary purines. The underlying ana Ziekenhuis, Dept. of Pediatrics, Rhenen, The Netherlands. mechanism possibly is an influence of dietary purines on A deficiency of purine nucleoside phosphorylase (PNP) was the formation of oxipurinol-ribotides. The latter being ex- detected in a three-year-old boy who was admitted for the creted as their corresponding aucleosides we first measured investigation of behaviour disorders and spastic diplegia. The the urinary excretion of oxipnrinol-7-riboside which was urinary excretion profile of oxypurines, analyzed by liquid found not to be altered by dietary purines. Therefore we chromatography, showed the presence of large amounts of searched for oxipurinol-1-riboside which had not yet been (deoxytinosine and (deoxy)guanosine together with a low uric detected in human urine. By HPM: we were able to demonstra- acid. The PNP activity in red blood cells and peripheral blood te the presence of this metabolite. -

IVPAIRED RENAL EXCRETION of HYPOXANTHINE Ihx) and Clinical
IVPAIRED RENAL EXCRETION OF HYPOXANTHINE IHx) AND RED BLOOD CELL MORPHOLOGY IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD): EFFECT OF OXYGEN THERAPY 122 VERSUS ALLOPURINOL. A MichBn, J Puig. P Crespo*. F Maccas", A GonzBlez, and J Ortiz. 'La Paz' Hospital, Clinical Biochemistry, Universidad Autbnoma, Madrid, Department of Internal Medicine, Universidad Aut6nomq Spain. Madrid, and 'Department of Histology, Granada, Spain. Most patients with primary gout show normal uric acid (UA) production rates and a relative underexcretion of UA. To examine Morphologic abnormalities in red blood cells depend on dlverse if the renal dysfunction for UA excretion in primary gout also factors which may include the intracellular centent of oxygen. affects the excretion of UA precursors, we compared the plasma Hypoxemia increases adenine nucleotide turnover and may influence concentrations and 24 hour urinary excretion of Hx and X in 10 erythrocyte morphology. To test this hypothesis in 7 patients normal srlojects, 15 patients with primary gout and UA under- with cliilically stable COPD we measured red cell ATP (iATP) excretioli and 10 patients with diverse enzyme deficiencies known levels by HPLC and observed erythrocyte morphology by scanning to overproduce UA. All subjects showed a creatinine clearance electron microscopy. Studies were carried out in 3 situations: above 80 ml/min/1.73 m2. Results (mean+SEM) were as follows basal state (mean+SEM; Pa02, 5823 mm Hg), after 7 days on oxygen therapy (Pa02, 79~4mm Hg; Pc0.01) and following a 7 day course PW URINE on allopurinol (300 mg/24 h; Pa02, 58+3 mm Hg). iATP concentra- UAHxX UA Hx X tions were similar in the 3 expermental conditions. -

Ultra Fast HPLC Food & Beverages YMC Ultraht
Application Guide Ultra Fast HPLC Food & Beverages YMC UltraHT HPLC Columns for Ultra Fast LC Nowadays the need for Ultra Fast LC and Rapid Resolution is still growing, especially in the pharmaceutical industry, due to the continuous demand for high throughput analysis in research & development and quality control. As a column and bulk media supplier with many years of practical chromato- graphic experience, YMC found it unacceptable that the use of novel sepa- ration media is often restricted to dedicated equipment and not applicable to the large installed base of “conventional” HPLC systems with a standard operating pressure rating of less than 400 bar. For this reason, specifications for YMC-UltraHT columns were designed to provide powerful chromatographic improvements, in terms of velocity and resolution, with conventional operating conditions as well as ultra-high pressure systems. Since YMC-UltraHT columns provides a substantially lower pressure drop than most competitive 2 µm or sub-2 µm media, high flow rates can be achieved without generating excessive back pressure and without the need for specialised equipment. For effective high throughput separations, YMC offer a wide range of high per- formance HPLC columns, which allow Ultra Fast analytical HPLC separations using conventional equipment. Due to the down-scalability of the majority of YMC’s stationary phases, the time needed for a single analysis can be reduced to less than 60 seconds, depending on the sample conditions. YMC-UltraHT Pro C18 (2μm, 12nm) 50 x 2.0mm ID �� 1. -

Effects of Allopurinol and Oxipurinol on Purine Synthesis in Cultured Human Cells
Effects of allopurinol and oxipurinol on purine synthesis in cultured human cells William N. Kelley, James B. Wyngaarden J Clin Invest. 1970;49(3):602-609. https://doi.org/10.1172/JCI106271. Research Article In the present study we have examined the effects of allopurinol and oxipurinol on thed e novo synthesis of purines in cultured human fibroblasts. Allopurinol inhibits de novo purine synthesis in the absence of xanthine oxidase. Inhibition at lower concentrations of the drug requires the presence of hypoxanthine-guanine phosphoribosyltransferase as it does in vivo. Although this suggests that the inhibitory effect of allopurinol at least at the lower concentrations tested is a consequence of its conversion to the ribonucleotide form in human cells, the nucleotide derivative could not be demonstrated. Several possible indirect consequences of such a conversion were also sought. There was no evidence that allopurinol was further utilized in the synthesis of nucleic acids in these cultured human cells and no effect of either allopurinol or oxipurinol on the long-term survival of human cells in vitro could be demonstrated. At higher concentrations, both allopurinol and oxipurinol inhibit the early steps ofd e novo purine synthesis in the absence of either xanthine oxidase or hypoxanthine-guanine phosphoribosyltransferase. This indicates that at higher drug concentrations, inhibition is occurring by some mechanism other than those previously postulated. Find the latest version: https://jci.me/106271/pdf Effects of Allopurinol and Oxipurinol on Purine Synthesis in Cultured Human Cells WILLIAM N. KELLEY and JAMES B. WYNGAARDEN From the Division of Metabolic and Genetic Diseases, Departments of Medicine and Biochemistry, Duke University Medical Center, Durham, North Carolina 27706 A B S TR A C T In the present study we have examined the de novo synthesis of purines in many patients. -

Allopurinol Metabolism in a Patient with Xanthine Oxidase Deficiency
Ann Rheum Dis: first published as 10.1136/ard.42.6.684 on 1 December 1983. Downloaded from Annals ofthe Rheumatic Diseases, 1983, 42, 684-686 Allopurinol metabolism in a patient with xanthine oxidase deficiency HISASHI YAMANAKA, KUSUKI NISHIOKA, TAKEHIKO SUZUKI,* AND KEIICHI KOHNO* From the Rheumatology Department, Clinical Research Centre, Tokyo Women's Medical College, 2-4-1, NS BLD, Nishishinjuku, Shinjukuku, Tokyo, Japan SUMMARY A patient with complete deficiency of xanthine oxidise would not be expected to oxidase allopurinol to oxipurinol if allopurinol did not have any alternative metabolic pathway. 400 mg of allopurinol was administered to a patient with xanthine oxidase deficiency, and plasma allopurinol, oxipurinol, hypoxanthine, and xanthine levels were determined serially by the use of high-perfornman9e liquid chromatography (HPLC). Plasma oxipurinol as well as allopurinol was increased after the administration of allopurinol, and oxipurinol reached a maximum level of 13 1 ,u.g/ml at 6 hours after the administration. This was the same pattern as that of normal controls. This result demonstrated the existence of some other oxidising enzyme of allopurinol than xanthine oxidase. Allopurinol (4-hydroxypyrazolo[3,4-d]pyrimidine) free diet. Creatinine clearance was 73 3 m/min, and is known as a potent inhibitor of xanthine oxidase no calculus was found in her urinary tract by abdomi- copyright. (EC 1.2.3.2). In addition allopurinol itself is nal plain x-ray film and drip infusion pyelography. metabolised in vivo by xanthine oxidase to oxipurinol The xanthine oxidase activity of the duodenal (4,6-dihydroxypyrazolo[ 3,4-d]pyrimidine, which mucosa, which was obtained by gastrofibroscopy, also inhibits xanthine oxidase. -

University of Huddersfield Repository
University of Huddersfield Repository Aburas, Omaro A Emhmed Investigation of aldehyde oxidase and xanthine oxidoreductase in rainbow trout (Oncorhynchus mykiss) Original Citation Aburas, Omaro A Emhmed (2014) Investigation of aldehyde oxidase and xanthine oxidoreductase in rainbow trout (Oncorhynchus mykiss). Doctoral thesis, University of Huddersfield. This version is available at http://eprints.hud.ac.uk/23543/ The University Repository is a digital collection of the research output of the University, available on Open Access. Copyright and Moral Rights for the items on this site are retained by the individual author and/or other copyright owners. Users may access full items free of charge; copies of full text items generally can be reproduced, displayed or performed and given to third parties in any format or medium for personal research or study, educational or not-for-profit purposes without prior permission or charge, provided: • The authors, title and full bibliographic details is credited in any copy; • A hyperlink and/or URL is included for the original metadata page; and • The content is not changed in any way. For more information, including our policy and submission procedure, please contact the Repository Team at: [email protected]. http://eprints.hud.ac.uk/ Investigation of aldehyde oxidase and xanthine oxidoreductase in rainbow trout (Oncorhynchus mykiss) Omaro Aburas B.Sc., M.Sc. Department of Chemical and Biological Science University of Huddersfield United Kingdom Thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy July 2014 Abstract Molybdo-flavoenzymes (MFEs), aldehyde oxidase (AOX) and xanthine oxidoreductase (XOR) are involved in the oxidation of N-heterocyclic compounds and aldehydes, many of which are environmental pollutants, drugs and vitamins. -

216 Miscellaneous Miscellaneous 217 Technical Information
216 Miscellaneous Miscellaneous 217 Technical Information Contents • Column Handling ........................................... 218-220 • Mobile Phases for RP-Columns ............................. 221 • HPLC Column Performance .................................. 222 • Inspection Reports ................................................. 223 • FAQ ................................................................ 224-227 • Troubleshooting ............................................. 228-231 • Basic Data .............................................................. 232 • Solvent Miscibility Table ......................................... 233 • Column Selection Guide ................................ 234-235 • Linear Scale-Up ..................................................... 236 • Preparative Column Selection Guide ..................... 237 • Substance Index ............................................ 238-246 Introduction Technical Information YMC produces chromatography packing materials and HPLC columns under very strict quality control procedures and delivers to customers only those products that pass the strict Quality Assurance tests prior to shipment. In order to ensure the best performance and long column life, the following instructions should be followed for all packed columns. * Application data by courtesy YMC Co., Ltd. 218 Miscellaneous Column Handling - shipping solvent - mobile phase considerations - mobile phase replacement and column cleaning - guard columns - column back pressures - temperature 1. Shipping solvent The -

The Role of Oxidative Stress in Hyperuricemia and Xanthine Oxidoreductase (XOR) Inhibitors
Hindawi Oxidative Medicine and Cellular Longevity Volume 2021, Article ID 1470380, 15 pages https://doi.org/10.1155/2021/1470380 Review Article The Role of Oxidative Stress in Hyperuricemia and Xanthine Oxidoreductase (XOR) Inhibitors Ning Liu ,1 Hu Xu,1 Qianqian Sun,1 Xiaojuan Yu,1 Wentong Chen,1 Hongquan Wei,1 Jie Jiang,1,2 Youzhi Xu ,1 and Wenjie Lu 1 1Basic Medical College, Anhui Medical University, Hefei 230032, China 2College of Pharmacy, Anhui Medical University, Hefei 230032, China Correspondence should be addressed to Youzhi Xu; [email protected] and Wenjie Lu; [email protected] Received 19 May 2020; Revised 5 March 2021; Accepted 12 March 2021; Published 27 March 2021 Academic Editor: Carlo G. Tocchetti Copyright © 2021 Ning Liu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Uric acid is the end product of purine metabolism in humans. Hyperuricemia is a metabolic disease caused by the increased formation or reduced excretion of serum uric acid (SUA). Alterations in SUA homeostasis have been linked to a number of diseases, and hyperuricemia is the major etiologic factor of gout and has been correlated with metabolic syndrome, cardiovascular disease, diabetes, hypertension, and renal disease. Oxidative stress is usually defined as an imbalance between free radicals and antioxidants in our body and is considered to be one of the main causes of cell damage and the development of disease. Studies have demonstrated that hyperuricemia is closely related to the generation of reactive oxygen species (ROS). -

Allopurinol Metabolism in a Patient
Ann Rheum Dis: first published as 10.1136/ard.42.6.684 on 1 December 1983. Downloaded from Annals ofthe Rheumatic Diseases, 1983, 42, 684-686 Allopurinol metabolism in a patient with xanthine oxidase deficiency HISASHI YAMANAKA, KUSUKI NISHIOKA, TAKEHIKO SUZUKI,* AND KEIICHI KOHNO* From the Rheumatology Department, Clinical Research Centre, Tokyo Women's Medical College, 2-4-1, NS BLD, Nishishinjuku, Shinjukuku, Tokyo, Japan SUMMARY A patient with complete deficiency of xanthine oxidise would not be expected to oxidase allopurinol to oxipurinol if allopurinol did not have any alternative metabolic pathway. 400 mg of allopurinol was administered to a patient with xanthine oxidase deficiency, and plasma allopurinol, oxipurinol, hypoxanthine, and xanthine levels were determined serially by the use of high-perfornman9e liquid chromatography (HPLC). Plasma oxipurinol as well as allopurinol was increased after the administration of allopurinol, and oxipurinol reached a maximum level of 13 1 ,u.g/ml at 6 hours after the administration. This was the same pattern as that of normal controls. This result demonstrated the existence of some other oxidising enzyme of allopurinol than xanthine oxidase. Allopurinol (4-hydroxypyrazolo[3,4-d]pyrimidine) free diet. Creatinine clearance was 73 3 m/min, and is known as a potent inhibitor of xanthine oxidase no calculus was found in her urinary tract by abdomi- copyright. (EC 1.2.3.2). In addition allopurinol itself is nal plain x-ray film and drip infusion pyelography. metabolised in vivo by xanthine oxidase to oxipurinol The xanthine oxidase activity of the duodenal (4,6-dihydroxypyrazolo[ 3,4-d]pyrimidine, which mucosa, which was obtained by gastrofibroscopy, also inhibits xanthine oxidase. -

Inhibitory Effects of Quercetin and Its Human and Microbial Metabolites on Xanthine Oxidase Enzyme
International Journal of Molecular Sciences Article Inhibitory Effects of Quercetin and Its Human and Microbial Metabolites on Xanthine Oxidase Enzyme Violetta Mohos 1,2, Attila Pánovics 1, Eszter Fliszár-Nyúl 1,2, Gabriella Schilli 3, Csaba Hetényi 3 , PˇremyslMladˇenka 4 , Paul W. Needs 5, Paul A. Kroon 5,Gábor Peth˝o 1,3 and Miklós Poór 1,2,* 1 Department of Pharmacology, University of Pécs, Faculty of Pharmacy, Szigeti út 12, H-7624 Pécs, Hungary; [email protected] (V.M.); [email protected] (A.P.); [email protected] (E.F.-N.); [email protected] (G.P.) 2 János Szentágothai Research Center, University of Pécs, Ifjúság útja 20, H-7624 Pécs, Hungary 3 Department of Pharmacology and Pharmacotherapy, University of Pécs, Medical School, Szigeti út 12, H-7624 Pécs, Hungary; [email protected] (G.S.); [email protected] (C.H.) 4 Department of Pharmacology and Toxicology, Faculty of Pharmacy in Hradec Králové, Charles University, Heyrovského 1203, 500 05 Hradec Králové, Czech Republic; [email protected] 5 Quadram Institute Bioscience, Norwich Research Park, Norwich NR4 7UA, UK; [email protected] (P.W.N.); [email protected] (P.A.K.) * Correspondence: [email protected]; Tel.: +36-536-000 (ext. 34646) Received: 10 May 2019; Accepted: 29 May 2019; Published: 31 May 2019 Abstract: Quercetin is an abundant flavonoid in nature and is used in several dietary supplements. Although quercetin is extensively metabolized by human enzymes and the colonic microflora, we have only few data regarding the pharmacokinetic interactions of its metabolites. -

Disorder Index
Disorder Index A AIP. See Acute intermittent porphyria (AIP) AARF domain-containing kinase 3 (ADCK3) defi ciency , 235 AKU. See Alkaptonuria (AKU) ABCB4 defi ciency , 557, 559, 564, 571 Alanine-glyoxylate aminotransferase (AGT) , 376, 466, 467, 471, ABCB11 defi ciency , 557, 564, 571 763, 772 ABCC2 defi ciency , 557, 564 Alanine-glyoxylate aminotransferase defi ciency (peroxisomal) , Abetalipoproteinemia (MTP) , 674, 678 467, 763 ABS. See Antley-Bixler syndrome (ABS) ALDOA defi ciency. See Aldolase A (ALDOA) defi ciency ACAD9 defi ciency. See Acyl-CoA dehydrogenase 9 (ACAD9) Aldoketoreductase 2 defi ciency, Backdoor pathway defect , 605 defi ciency Aldolase A (ALDOA) defi ciency , 269, 821 N -Acetyl-alpha- d -glucosaminidase defi ciency , 450 Aldolase B (ALDOB) defi ciency , 268, 820 N -Acetylaspartic aciduria , 143 Aldosterone synthase defi ciency , 605 Acetyl-CoA alpha-glucosaminide acetyltransferase defi ciency , 450 ALG1-CDG. See Mannosyltransferase 1 defi ciency congenital N -Acetylgalactosamine-4-sulphatase defi ciency , 450 disorders of glycosylation (ALG1-CDG) N -Acetylgalactosamine-6-sulphatase defi ciency , 450 ALG 2-CDG. See Mannosyltransferase 2 defi ciency congenital N -Acetylglucosamine-1-phosphotransferase (alpha/beta) disorders of glycosylation (ALG 2-CDG) defi ciency , 402 ALG3-CDG. See Mannosyltransferase 6 defi ciency congenital N -Acetylglucosamine-1-phosphotransferase (gamma) defi ciency , 402 disorders of glycosylation (ALG3-CDG) N -Acetylglucosamine-6-sulphatase defi ciency , 450 ALG6-CDG. See Glucosyltransferase 1 defi ciency, congenital N -Acetylglucosaminyltransferase 2 congenital defects of glycosylation disorders of glycosylation (ALG6-CDG) (MGAT2-CDG) , 484–486, 489 ALG 8-CDG. See Glucosyltransferase 2 defi ciency, congenital N -Acetylglucosaminyltransferase 2 defi ciency-CDG-IIa , 486, 496 disorders of glycosylation (ALG 8-CDG) N -Acetylglutamate synthase defi ciency , 49, 51, 715, 818 ALG9-CDG. -

Studies on the Coordinate Activity and Lability of Orotidylate
Studies on the coordinate activity and lability of orotidylate phosphoribosyltransferase and decarboxylase in human erythrocytes, and the effects of allopurinol administration Richard M. Fox, … , Margaret H. Wood, William J. O'Sullivan J Clin Invest. 1971;50(5):1050-1060. https://doi.org/10.1172/JCI106576. Research Article A coordinate relationship between the activities of two sequential enzymes in the de novo pyrimidine biosynthetic pathway has been demonstrated in human red cells. The two enzymes, orotidylate phosphoribosyltransferase and decarboxylase are responsible for the conversion of orotic acid to uridine-5′-monophosphate. Fractionation of red cells, on the basis of increase of specific gravity with cell age, has revealed that these two enzymes have a marked but equal degree of lability in the ageing red cell. It is postulated that orotidylate phosphoribosyltransferase and decarboxylase form an enzyme- enzyme complex, and that the sequential deficiency of these two enzymes in hereditary orotic aciduria may reflect a structural abnormality in this complex. In patients receiving allopurinol, the activities of both enzymes are coordinately increased, and this increase appears to be due, at least in part, to stabilization of both orotidylate phosphoribosyltransferase and decarboxylase in the ageing red cell. Allopurinol ribonucleotide is an in vitro inhibitor of orotidine-5′-monophosphate decarboxylase and requires the enzyme hypoxanthineguanine phosphoribosyltransferase for its synthesis. However, the administration of allopurinol to patients lacking this enzyme results in orotidinuria and these patients have elevated orotidylate phosphoribosyltransferase and decarboxylase activities in their erythrocytes. Evidence is presented that the chief metabolite of allopurinol, oxipurinol, with a 2,4-diketo pyrimidine ring is capable of acting as an analogue of orotic acid.