Infestation of Rhyzopertha Dominica First Instars on Different Classes of Wheat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Plant Oils on the Infestation of Rhyzopertha Dominica (Fab.) in Wheat, Triticum Aestivum Linn
JOURNAL OF PLANT PROTECTION RESEARCH Vol. 53, No. 3 (2013) DOI: 10.2478/jppr-2013-0045 EFFECT OF PLANT OILS ON THE INFESTATION OF RHYZOPERTHA DOMINICA (FAB.) IN WHEAT, TRITICUM AESTIVUM LINN. Kailash Chand Kumawat*, Bhanwar Lal Naga Department of Entomology, Shri Karan Narendra College of Agriculture (SKRAU), Jobner (Rajasthan)-303329, India Received: April 20, 2012 Accepted: August 8, 2013 Abstract: Six oil treatments, viz., Neem (Azadirachta indica A. Juss), Castor (Ricinus communis), Karanj (Pongamia pinnata), mustard (Brassica juncea), Eucalyptus (Eucalyptus melanophloia) and Taramira (Eruca sativa) were evaluated at three dose levels (0.1, 0.5, and 1.0% v/w) against the lesser grain borer, Rhyzopertha dominica (Fab.) infesting wheat, Triticum aestivum Linn. An untreated check (the control) was maintained for comparison. The maximum protection was provided by Neem oil at 1.0 per cent (no adult emerged up to 270 days) followed by castor oil and Taramira oil at 1.0 per cent (no adult emerged up to 90 days of treatment). The maximum number of adults were recorded in the grain treated with Eucalyptus oil used at 0.1 per cent (9.3–22.0), Karanj oil at 0.1 per cent (6.0–20.7), and castor oil at 0.1 per cent (2.0–23.0). The maximum grain damage was recorded with use of Eucalyptus oil at 0.1 per cent (28.7–64.7), Karanj oil at 0.1 per cent (18.7–60.0%), and Eucalyptus at 0.5 per cent (18.0–58.0%). No grain damage was recorded in 1.0 per cent Neem oil-treated grain, for up to 270 days. -

Rhyzopertha Dominica (Coleoptera: Bostrichidae) Infestation on Seeds of Sorghum Drummondii (Poaceae) in Packages Sold in Retail Stores
Revista Brasileira de Entomologia 65(2):e20200129, 2021 Rhyzopertha dominica (Coleoptera: Bostrichidae) infestation on seeds of Sorghum drummondii (Poaceae) in packages sold in retail stores David Lopes Teixeira1* , Pedro Guilherme Lemes1, Thiago Gomes dos Santos Braz2, Germano Leão Demolin Leite1 , José Cola Zanuncio3 1Universidade Federal de Minas Gerais, Instituto de Ciências Agrárias, Laboratório de Entomologia Aplicada à Área Florestal, Montes Claros, MG, Brasil. 2Universidade Federal de Minas Gerais, Instituto de Ciências Agrárias, Laboratório de Fenotipagem de Plantas, Montes Claros, MG, Brasil. 3Universidade Federal de Viçosa, Departamento de Entomologia/BIOAGRO, Viçosa, MG, Brasil. ARTICLE INFO ABSTRACT Article history: Insect damage to stored seeds is a challenge. Rhyzopertha dominica (Fabricius, 1792) (Coleoptera: Bostrichidae) is a Received 28 December 2020 major pest of seeds and grains in the world, but without record in seeds of the sudangrass (Sorghum drummondii Accepted 27 April 2021 (Poaceae)). The objective of this work was to report, for the first time, the occurrence and damage by R. dominica Available online 21 May 2021 in S. drummondii seeds, sold in sealed packages in retail market. Four samples with 500 seeds each and without Associate Editor: Regiane Cristina Bueno adult insects were separated from a package. The initial weight was obtained with a precision scale and the seeds were stored. The number of adult insects, the weight loss and the infestation rate of the seeds were evaluated 60 days later and the average between samples used to extrapolate the damage per package. An adult of R. dominica, Keywords: on average, was obtained for each seven seeds and 54.06% of the seeds were damaged, with an average weight Annual pasture loss of 36.09%. -

Novel Bacteriocyte-Associated Pleomorphic Symbiont of the Grain
Okude et al. Zoological Letters (2017) 3:13 DOI 10.1186/s40851-017-0073-8 RESEARCH ARTICLE Open Access Novel bacteriocyte-associated pleomorphic symbiont of the grain pest beetle Rhyzopertha dominica (Coleoptera: Bostrichidae) Genta Okude1,2*, Ryuichi Koga1, Toshinari Hayashi1,2, Yudai Nishide1,3, Xian-Ying Meng1, Naruo Nikoh4, Akihiro Miyanoshita5 and Takema Fukatsu1,2,6* Abstract Background: The lesser grain borer Rhyzopertha dominica (Coleoptera: Bostrichidae) is a stored-product pest beetle. Early histological studies dating back to 1930s have reported that R. dominica and other bostrichid species possess a pair of oval symbiotic organs, called the bacteriomes, in which the cytoplasm is densely populated by pleomorphic symbiotic bacteria of peculiar rosette-like shape. However, the microbiological nature of the symbiont has remained elusive. Results: Here we investigated the bacterial symbiont of R. dominica using modern molecular, histological, and microscopic techniques. Whole-mount fluorescence in situ hybridization specifically targeting symbiotic bacteria consistently detected paired bacteriomes, in which the cytoplasm was full of pleomorphic bacterial cells, in the abdomen of adults, pupae and larvae, confirming previous histological descriptions. Molecular phylogenetic analysis identified the symbiont as a member of the Bacteroidetes, in which the symbiont constituted a distinct bacterial lineage allied to a variety of insect-associated endosymbiont clades, including Uzinura of diaspidid scales, Walczuchella of giant scales, Brownia of root mealybugs, Sulcia of diverse hemipterans, and Blattabacterium of roaches. The symbiont gene exhibited markedly AT-biased nucleotide composition and significantly accelerated molecular evolution, suggesting degenerative evolution of the symbiont genome. The symbiotic bacteria were detected in oocytes and embryos, confirming continuous host–symbiont association and vertical symbiont transmission in the host life cycle. -

Effect of Host Age on Progeny Production of Theocolax Elegans
Kasetsart J. (Nat. Sci.) 48 : 587 - 597 (2014) Effect of Host Age on Progeny Production of Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae) Reared on Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae) Bonginkhosi E. Dlamini* and Weerawan Amornsak ABSTRACT Five host ages of Maize weevil, Sitophilus zeamais (Motschulsky) reared on brown rice were examined for progeny production of Theocolax elegans (Westwood). Brown rice kernels infested with S. zeamais were exposed to a mated female of T. elegans after 13, 15, 17, 19 and 21 d following S. zeamais introduction. Host stages were determined by measuring head-capsule widths from all the host ages. There was a signifi cant difference (P < 0.05) in T. elegans progeny production among the different host ages. Total progeny, total female progeny and total male progeny produced by 19-day-old S. zeamais larvae were signifi cantly higher (P < 0.05) compared to the other host ages. Progeny of T. elegans raised on 19-day-old S. zeamais larvae had a higher female to male ratio compared to the other host ages. Sitophilus zeamais larvae after 13, 15–17 and 19–21 d were found to be second, third and fourth instars, respectively. It was concluded that T. elegans can develop on the second, third and fourth instar larvae of S. zeamais. However, 19-day-old (fourth instar) S. zeamais larvae produced more T. elegans progeny with a higher female to male ratio. Keywords: Sitophilus zeamais, Theocolax elegans, host ages, progeny production, parasitoid INTRODUCTION have adverse effects on consumers and long-term residual effect on the environment (Phillips, 1997; Rice and maize are important food Charlet et al., 2002; Flinn and Hagstrum, 2002; crops of many countries of the world and are Bale et al., 2007), while biological control agents grown for grain which is stored because it cannot have no adverse effects on consumers or the be distributed or consumed immediately (Flinn environment (Flinn, 1998; Tefera et al., 2010). -

Influence of Wheat Cultivar, Temperature, and Theocolax
INFLUENCE OF WHEAT CULTIVAR, TEMPERATURE, AND THEOCOLAX ELEGANS (HYMENOPTERA: PTEROMALIDAE) ON RHYZOPERTHA DOMINICA (COLEOPTERA: BOSTRICHIDAE) DEVELOPMENT BY MICHAEL D. TOEWS Bachelor ofScience Fort Hays State University Hays, Kansas 1995 Submitted to the Faculty ofthe Graduate College ofthe Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE May 1998 INFLUENCE OF WHEAT CULTrVAR, TEMPERATURE, AND THEOCOLAX ELEGANS (HYMENOPTERA: PTEROMALIDAE) ON RHYZOPERTHA DOMINICA (COLEOPTERA: BOSTRICHIDAE) DEVELOPMENT Thesis Approved: ~~~ ~JJ.~D~~_ /~,) 6L~fN-r , ean ofthe Graduate College n PREFACE The first chapter ofthis thesis is a literature review focused on issues in stored wheat. Also induded in chapter one is a review ofthe lesser grain borer, the parasitoid Theocolax elegans, and interactions among the trophic levels in my research. Subsequent chapters are formal papers representing my M.S. research project and are written in compliance with the publication policies and guidelines for manuscript preparation with the Entomological Society ofAmerica. The completion ofthis degree would not have been possible without the guidance ofmany people. I would like to express my sincere appreciation to my graduate advisor, Dr. Gemt Cuperus, for his assistance and direction. My co-advisor, Dr. Tom Phillips, provided a great deal ofpractical assistance and advice while also housing me in his laboratory space. This research project greatly benefited from the insight offered by Dr. Richard Berberet and Dr. Phillip Mulder. Special appreciation is directed toward Dr. Mark Payton who answered many questions and assisted me with the design and analysis of each experiment. I wish to extend special thanks to Edmond Bonjour for his proofreading and example throughout all phases ofmy degree. -

Grain Borer (Rhyzopertha Dominica) (Coleoptera: Bostrichidae)
Comp. Biochem. PhysioL Vol. 106B, No. 2, pp. 407-414, 1993 0305-0491/93 $6.00 + 0.00 Printed in Great Britain Pergamon Press Ltd CUTICULAR HYDROCARBONS OF WINGED AND WINGLESS MORPHS OF THE ECTOPARASITOID CHOETOSPILA ELEGANS WESTWOOD (HYMENOPTERA: PTEROMALIDAE) AND ITS HOST, LARVAL LESSER GRAIN BORER (RHYZOPERTHA DOMINICA) (COLEOPTERA: BOSTRICHIDAE) RALPH W. HOWARD* and YONGSHENG LIANGt~ USDA Agriculture Research Service, Grain Marketing Research Laboratory, 1515 College Avenue, Manhattan, KS 66502, U.S.A. (Tel. 913-776-2706; Fax 913-776-2792) tChengdu Grain Storage Research Institute, Ministry of Commerce, 95 Huapalfang St., Chengdu, Sichuan Province, People's Republic of China (Received 9 February 1993; accepted 12 March 1993) Abstract--1. The cuticular hydrocarbons from winged and wingless morphs of Choetospila elegans, a larval ectoparasite of several internal-feeding stored product beetle pests, were characterized. 2. All four morphs share the same cuticular hydrocarbons, with the major components being n-alkanes (C21-C33). 3. The minor components are 3-, ! 1- and 13-methyl branched alkanes and Z-10-monoenes. 4. Two-way analysis of variance (sex and wing morph) shows that males and females have the same profiles, whereas four components showed significant differences between wingtype morphs. 5. Only one of these four hydrocarbons (n-C31) was a major component. 6. The cuticular hydrocarbons of larvae of the lesser grain borer, Rhyzopertha dominica, were also characterized. 7. Although the beetles' major components were the same n-alkanes as those found on the adult parasites, their minor components were different. 8. Thus, the beetle larvae have no alkenes, but instead have 3-, 11-, 13- and 15-methylbranched alkanes, as well as a series of 11,15-dimethylalkanes. -

Toxicity of Some Plant Powders to Lesser Grain Borer (Rhyzopertha Dominica (Fab.); Coleoptera: Bostrichidea) Infesting Stored Sorghum
International Journal of Advanced Academic Research | Sciences, Technology and Engineering | ISSN: 2488-9849 Vol. 5, Issue 12 (December 2019) TOXICITY OF SOME PLANT POWDERS TO LESSER GRAIN BORER (RHYZOPERTHA DOMINICA (FAB.); COLEOPTERA: BOSTRICHIDEA) INFESTING STORED SORGHUM. USMAN, M.1; MAJEED, Q.2; ABDULLAHI, K.3; SOKOTO, M. B.4; MAINASARA, H.5 AND MUSA, A.6 1Biology Department, College of Education (Tech.), Lafiagi, Kwara State, Nigeria. 2Department of Biological Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria. 3Department of Biological Sciences, Federal University Gusau, Zamfara State, Nigeria. 4Department of Crop Science, Usmanu Danfodiyo University Sokoto, Nigeria. 5Department of Science Laboratory Technology, College of Agriculture and animal Sciences, Bakura, Zamfara State, Nigeria. 6Department of Agricultural Science Education, College of Education (Tech.), Lafiagi, Kwara State, Nigeria. ABSTRACT Powders of neem seed Kernel (Azadirachta indica A. JUSS.), bush tea (Hyptis suaveolens L. poit.), Jatropha (Jatropha curcas L.), bitter melon (Momordica charantia L.) and Mahogany (khaya senegalensis Desr.) were applied at 0.5, 1.0. 1.5 and 2.0g per 20g of sorghum grain. Cypermethrin (2.0%) as control check was applied at 0.12g/20g and evaluated for toxicity against Rhyzopertha dominica Fab. on stored sorghum grains. Jatropha leaf, Mahogany stem bark and neem seed kernel powders at all concentrations recorded 76.67% and above mortality of adult R. dominica as cypermethrin. A. indica, J. curcas and K. senegalensis suppressed oviposition and reduced adult emergence of F2, offsprings. Sorghum grain damage and weight loss were highly reduced and so protect by A indica at 0.5g, while J.curcas and K. senegalensis were both effective at 1.0g and 2.0g for grain damage and weight loss prevention respectively up to 150 days of storage. -

Prostephanus Truncatus and Teretrius Nigrescens Demonstrated Bya Cheap and Simple Pheromone-Baited Trap Designed to Segregate Catches with Time L.A
ARTICLE IN PRESS Journal of Stored Products Research 40 (2004) 227–232 Short communication Flight behaviour of Prostephanus truncatus and Teretrius nigrescens demonstrated bya cheap and simple pheromone-baited trap designed to segregate catches with time L.A. Birkinshawa, R.J. Hodgesa,*, S. Addob a Natural Resources Institute, University of Greenwich, Chatham Maritime, Kent ME4 4TB, UK b Post-harvest Management Division, Ministry of Food and Agriculture, PO Box HP 165, Ho, Ghana Accepted 24 September 2002 Abstract The storage pest Prostephanus truncatus (Horn) (Coleoptera: Histeridae) and its predator Teretrius nigrescens (Lewis) (Coleoptera: Histeridae) are both known to disperse byflight. The pattern of flight activityof the two beetles in Ghana, across 11 months of the year, was investigated using a novel flight trap that separates catch at 3-h intervals. Prostephanus truncatus showed most flight activityaround dusk with a smaller peak around dawn. Teretrius nigrescens had a strong diurnal peak. There were considerable differences in catch of both species during the year and when catch was low the peaks in activity were also less distinct. r 2003 Elsevier Science Ltd. All rights reserved. Keywords: Flight trapping; Flight behaviour; Pheromone trap 1. Introduction It is well known that insects favour particular times of dayfor flight. Several insect pests of stored products, e.g. Rhyzopertha dominica (F.) (Barrer et al., 1993), Sitophilus zeamais Motschulskyand Ephestia cautella (Walker) (Giles, 1969), show mid- to late afternoon peaks in flight activity. This behaviour can be studied using traps such as the Johnson–Taylor suction trap (Burkard Ltd, UK) that separate catch according to time of day. -

Weight Losses of Wheat Grains Caused by Psocid Infestation (Liposcelis Bostrychophila: Liposcelididae: Psocoptera)
Plant Protection Science – 2002 Vol. 38, No. 3: 103–107 Weight Losses of Wheat Grains Caused by Psocid Infestation (Liposcelis bostrychophila: Liposcelididae: Psocoptera) ZUZANA KUČEROVÁ Department of Stored-Product Pest Control – Research Institute of Crop Production, Prague-Ruzyně, Czech Republic Abstract KUČEROVÁ Z. (2002): Weight losses of wheat grains caused by psocid infestation (Liposcelis bostrychophila: Liposcelidi- dae: Psocoptera). Plant Protect. Sci., 38: 103–107. Psocids are commonly found to be a persistent pest in structures of grain stores. Grain residues are potential pest reservoirs that serve as sources of grain re-infestation. Weight losses caused by psocids on broken wheat kernels were measured. Average weight loss of grain samples was 9.7% after 3 months of Liposcelis bostrychophila infestation. The weight losses were positively correlated with progeny production. Keywords: Liposcelis bostrychophila; Psocoptera; stored grain; weight losses Stored product losses resulting from insect infestation sociated with this problem. Certain psocids (liposcelids) are extensive. They are usually associated with the oc- are allergenic to susceptible people (TURNER et al. 1996). currence of internal feeders such as Rhyzopertha domi- Heavy infestations of these insects can cause discomfort nica (STEJSKAL et al. 1999) or Sitophilus sp. (KUČEROVÁ amongst store workers, especially in tropical countries & STEJSKAL 1994). But many of the so-called second- (MILLS et al. 1992). Presence of insects in a commodity ary pests can also play an important role in directly dam- can cause its rejection for export. Furthermore, psocid aging stored products. infestation can also cause physical damage to stored grain. Stored-product psocids used to be regarded as a nui- Psocids are able to readily feed even on grain germ and sance, feeding especially on moulds. -

(Coleoptera: Bostrichidae) Fed on Various Barley Cultivars
Journal of Insect Science, (2018) 18(2): 31; 1–9 doi: 10.1093/jisesa/iey022 Research Comparison of Life Table Parameters and Digestive Physiology of Rhyzopertha dominica (Coleoptera: Bostrichidae) Fed on Various Barley Cultivars Maryam Nemati-Kalkhoran, Jabraeil Razmjou,1 Ehsan Borzoui, and Bahram Naseri Department of Plant Protection, Faculty of Agriculture and Natural Resources, University of Mohaghegh Ardabili, Ardabil, Iran and 1Corresponding author, e-mail: [email protected] Subject Editor: Christos Athanassiou Received 25 December 2017; Editorial decision 18 February 2018 Abstract In this study, the effect of 20 barley cultivars were evaluated on the life table parameters and digestive enzymatic activity of the lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) under laboratory conditions (28 ± 1°C, 60 ± 5% RH, and a photoperiod of 16:8 (L:D) h). Among barley cultivars tested developmental time of R. dominica immature stages was longest on cultivar Bahman (61.00) and shortest on Mahoor (46.60 d). The lowest realized fecundities were recorded for insects reared on cultivar Bahman (217.60); and the highest ones were observed for insects reared on Sahra (348.05 eggs/female). The net reproductive rate (R0) was significantly affected by various barley cultivars being lowest on cultivar Bahman (53.98) and highest on Mahoor (146.79 offspring/ female). Records for intrinsic rates of increase (rm) were lowest on cultivar Dasht (0.043) and the highest on Mahoor (0.066 day−1). The highest levels of amylolytic and proteolytic activity were recorded on cultivars Mahoor and EBYT- 92-10, respectively. By contrast, the insects reared on cultivars Dasht had the lowest levels of α-amylase and general protease activity. -

Thermal Death Kinetics of Cryptolestes Pusillus (Schonherr), Rhyzopertha Dominica (Fabricius), and Tribolium Confusum (Jacquelin Du Val) Using a Heating Block System
insects Article Thermal Death Kinetics of Cryptolestes pusillus (Schonherr), Rhyzopertha dominica (Fabricius), and Tribolium confusum (Jacquelin du Val) Using a Heating Block System Lixia Hou 1, Yi Wu 2 and Shaojin Wang 1,3,* 1 College of Mechanical and Electronic Engineering, Northwest A&F University, Yangling, Shaanxi 712100, China; [email protected] 2 Academy of State Administration of Grain, Beijing 100037, China; [email protected] 3 Department of Biological Systems Engineering, Washington State University, 213 L.J. Smith Hall, Pullman, WA 99164-6120, USA * Correspondence: [email protected]; Tel.: +86-29-8709-2391; Fax: +86-29-8709-1737 Received: 24 March 2019; Accepted: 24 April 2019; Published: 26 April 2019 Abstract: Thermal treatment has been extensively used to control pests in stored grains for a long time. The objective of this study was to analyze thermal death kinetics of adult flat grain beetle, Cryptolestes pusillus (Schonherr), lesser grain borer, Rhyzopertha dominica (Fabricius), and confused flour beetle, Tribolium confusum (Jacquelin du Val), using a heating block system (HBS), at temperatures of 46, 48, 50, and 52 ◦C for C. pusillus and T. confusum, and 48, 50, 52, and 54 ◦C for R. dominica with a heating rate of 5 ◦C/min. Thermal death curves of those three insects followed a 0th-order reaction model. Complete mortality of C. pusillus, R. dominica, and T. confusum were observed after exposure to 1.4, 5.0, and 0.9 min at 52, 54 and 52 ◦C, respectively. The thermal death activation energy for controlling C. pusillus, R. dominica, and T. confusum was 689.91, 380.88, and 617.08 kJ/mol with z values of 2.88, 5.18, and 3.22 ◦C, respectively. -
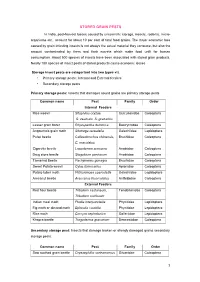
Stored Grain Pests
STORED GRAIN PESTS In India, post-harvest losses caused by unscientific storage, insects, rodents, micro- organisms etc., account for about 10 per cent of total food grains. The major economic loss caused by grain infesting insects is not always the actual material they consume, but also the amount contaminated by them and their excreta which make food unfit for human consumption. About 500 species of insects have been associated with stored grain products. Nearly 100 species of insect pests of stored products cause economic losses Storage insect pests are categorized into two types viz. • Primary storage pests : Internal and External feeders • Secondary storage pests Primary storage pests: Insects that damages sound grains are primary storage pests Common name Pest Family Order Internal Feeders Rice weevil Sitophilus oryzae, Curculionidae Coleoptera S. zeamais, S. granarius Lesser grain borer Rhyzopertha dominica Bostrychidae Coleoptera Angoumois grain moth Sitotroga cerealella Gelechiidae Lepidoptera Pulse beetle Callosobruchus chinensis, Bruchidae Coleoptera C. maculatus Cigarette beetle Lasioderma sericorne Anobiidae Coleoptera Drug store beetle Stegobium paniceum Anobiidae Coleoptera Tamarind Beetle Pachymeres gonagra Bruchidae Coleoptera Sweet Potato weevil Cylas formicarius Apionidae Coleoptera Potato tuber moth Phthorimoea operculella Gelechiidae Lepidoptera Arecanut beetle Araecerus fasciculatus Anthribidae Coleoptera External Feeders Red flour beetle Tribolium castaneum, Tenebrionidae Coleoptera Tribolium confusum Indian meal