Neuroanatomical Structure of the Spinner Dolphin (Stenella Longirostris Orientalis) Brain from Magnetic Resonance Images
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morphology and Distribution of the Spinner Dolphin, Stenella
J. CETACEAN RES. MANAGE. 1(2):167- 177 , 1999 167 Morphology and distribution of the spinner dolphin,Stenella longirostris, rough-toothed dolphin,Steno bredanensis and melon-headed whale,Peponocephala electra, from waters off the Sultanate of Oman1 Koen Van W aerebeek*, M ichael G allagheri Robert Baldwin Vassili Papastavrou§ and Samira M ustafa A l - L a w a t i 1 Contact e-mail: [email protected] ABSTRACT The morphology of three tropical delphinids from the Sultanate of Oman and their occurrence in the Arabian Sea are presented. Body lengths of four physically mature spinner dolphins (three males) ranged from 154-178.3cm (median 164.5cm), i.e. smaller than any known stock of spinner dolphins, except the dwarf forms from Thailand and Australia. Skulls of Oman spinner dolphins (n = 10) were practically indistinguishable from those of eastern spinner dolphins ( Stenella longirostris orientalis) from the eastern tropical Pacific, but were considerably smaller than skulls of populations of pantropical ( Stenella longirostris longirostris) and Central American spinner dolphins (Stenella longirostris centroamericana). Two colour morphs (CM) were observed. The most common (CM1) has the typical tripartite pattem of the pantropical spinner dolphin. A small morph (CM2), so far seen mostly off Muscat, is characterised by a dark dorsal overlay obscuring most of the tripartite pattern and by a pinkish or white ventral field and supragenital patch. Two skulls were linked to a CM1 colour morph, the others were undetermined. It is concluded that Oman spinner dolphins should be treated as a discrete population, morphologically distinct from all known spinner dolphin subspecies. -

Convention on Migratory Species
Distr: General CONVENTION ON CMS/PIC/MoS3/Inf.3.1.4 MIGRATORY 6 September 2012 SPECIES Original: English THIRD MEETING OF THE SIGNATORIES TO THE MEMORANDUM OF UNDERSTANDING FOR THE CONSERVATION OF CETACEANS AND THEIR HABITATS IN THE PACIFIC ISLANDS REGION Noumea, New Caledonia, 8 September 2012 Agenda Item 3.1 BUILDING ON THE LOCAL KNOWLEDGE OF WHALES AND DOLPHINS ALONG THE SOUTHERN COAST OF UPOLU AND THE NORTHWESTERN COAST OF SAVAI’I For reasons of economy, this document is printed in a limited number, and will not be distributed at the meeting. Delegates are kindly requested to bring their copy to the meeting and not to request additional copies. BUILDING ON THE LOCAL KNOWLEDGE OF WHALES AND DOLPHINS ALONG THE SOUTHERN COAST OF UPOLU AND THE NORTHWESTERN COAST OF SAVAI’I 20TH SEPTEMBER – 29TH OCTOBER 2010 Prepared by: Juney Ward, Malama Momoemausu, Pulea Ifopo, Titimanu Simi, Ieru Solomona1 1. Division of Environment & Conservation Staff, Ministry of Natural Resources & Environment TABLE OF CONTENTS 1. INTRODUTION ..................................................................................... 2 2. SURVEY OBJECTIVES .......................................................................... 3 3. METHODOLOGY ................................................................................ 3 - 4 a. Study area ................................................................................ 3 b. Data collection ........................................................................ 4 c. Photo-identification ................................................................. -

ARTICLE in PRESS Aquatic Mammals 2019, 45(3), Xxx-Xxx, DOI 10.1578/AM.45.3.2019.Xxx
ARTICLE IN PRESS Aquatic Mammals 2019, 45(3), xxx-xxx, DOI 10.1578/AM.45.3.2019.xxx Incidence of Odontocetes with Dorsal Fin Collapse in Maui Nui, Hawaii Stephanie H. Stack, Jens J. Currie, Jessica A. McCordic, and Grace L. Olson Pacific Whale Foundation, 300 Ma‘alaea Road, Suite 211, Wailuku, HI 96793, USA E-mail: [email protected] Abstract Introduction We examined the incidence of bent or col- A variety of dorsal fin injuries have been docu- lapsed dorsal fins of eight species of odontocetes mented in several odontocete species worldwide, observed in the nearshore waters of Maui Nui, but laterally bent or collapsed dorsal fins are a rel- Hawaii. Between 1995 and 2017, 1,312 distinc- atively uncommon occurrence (Baird & Gorgone, tive individual odontocetes were photographically 2005; Van Waerebeek et al., 2007; Luksenburg, documented. Our photo-identification catalogs in- 2014). Dorsal fin collapse is rare in wild popu- clude 583 spinner dolphins (Stenella longirostris lations of odontocetes, with published rates longirostris), 164 bottlenose dolphins (Tursiops generally less than 1%, if at all present (Baird truncatus), 132 short-finned pilot whales (Glo- & Gorgone, 2005). Exceptions to this are well- bicephala macrorhynchus), 253 pantropical studied populations of killer whales (Orcinus spotted dolphins (Stenella attenuata), 82 false orca) and the main Hawaiian Islands’ popula- killer whales (Pseudorca crassidens), 70 melon- tion of false killer whales (Pseudorca crassidens) headed whales (Peponocephala electra), 15 (Visser, 1998; Alves et al., 2017). A recent pub- pygmy killer whales (Feresa attenuata), and 13 lication by Alves et al. (2017) documented 17 rough-toothed dolphins (Steno bredanensis). -

Nai'a Or Spinner Dolphin
Courtesy Cascadia Research. Photo taken under MMPA Scientific Research Permit No. 731 © Dan McSweeney Marine Mammals Nai‘a or Spinner dolphin Stenella longirostris SPECIES STATUS: IUCN Red List - Lower Risk/ Conservation Dependent SPECIES INFORMATION: Nai‘a or spinner dolphins (Stenella longirostris) congregate into large groups and swim offshore to depths of 200 to 300 meters (650 to 1,000 feet) to feed on mesopelagic prey that includes squid, fish and shrimp. Although in large groups, they also feed in cooperative pairs or groups of pairs offshore. This foraging begins in late afternoon and continues throughout the night as the “deep scattering layer” moves closer to the surface. Recent research shows that this food source is close to shore early in the night so the spinner dolphins follow them inshore for awhile. During the day, they expend less energy resting or socializing in nearshore, shallow waters such as bays and lagoons surrounded by reef. They also may stay in their nearshore habitat to avoid predators such as sharks and killer whales. The change in group size from daytime activities to nighttime feeding is unique to Hawaii’s spinner dolphins. Although spinner dolphins are able to give birth at any time during the year, they typically show one or more seasonal peaks. Multiple males may mate with one female in short, consecutive intervals. Gestation lasts approximately ten and a half months and lactation occurs for one to two years. The calving interval is approximately three years. Additionally, the spinner dolphin is very notable for its ability to leap high out of the water while also spinning multiple times on its longitudinal axis. -

Marine Mammal Taxonomy
Marine Mammal Taxonomy Kingdom: Animalia (Animals) Phylum: Chordata (Animals with notochords) Subphylum: Vertebrata (Vertebrates) Class: Mammalia (Mammals) Order: Cetacea (Cetaceans) Suborder: Mysticeti (Baleen Whales) Family: Balaenidae (Right Whales) Balaena mysticetus Bowhead whale Eubalaena australis Southern right whale Eubalaena glacialis North Atlantic right whale Eubalaena japonica North Pacific right whale Family: Neobalaenidae (Pygmy Right Whale) Caperea marginata Pygmy right whale Family: Eschrichtiidae (Grey Whale) Eschrichtius robustus Grey whale Family: Balaenopteridae (Rorquals) Balaenoptera acutorostrata Minke whale Balaenoptera bonaerensis Arctic Minke whale Balaenoptera borealis Sei whale Balaenoptera edeni Byrde’s whale Balaenoptera musculus Blue whale Balaenoptera physalus Fin whale Megaptera novaeangliae Humpback whale Order: Cetacea (Cetaceans) Suborder: Odontoceti (Toothed Whales) Family: Physeteridae (Sperm Whale) Physeter macrocephalus Sperm whale Family: Kogiidae (Pygmy and Dwarf Sperm Whales) Kogia breviceps Pygmy sperm whale Kogia sima Dwarf sperm whale DOLPHIN R ESEARCH C ENTER , 58901 Overseas Hwy, Grassy Key, FL 33050 (305) 289 -1121 www.dolphins.org Family: Platanistidae (South Asian River Dolphin) Platanista gangetica gangetica South Asian river dolphin (also known as Ganges and Indus river dolphins) Family: Iniidae (Amazon River Dolphin) Inia geoffrensis Amazon river dolphin (boto) Family: Lipotidae (Chinese River Dolphin) Lipotes vexillifer Chinese river dolphin (baiji) Family: Pontoporiidae (Franciscana) -

Review of Small Cetaceans. Distribution, Behaviour, Migration and Threats
Review of Small Cetaceans Distribution, Behaviour, Migration and Threats by Boris M. Culik Illustrations by Maurizio Wurtz, Artescienza Marine Mammal Action Plan / Regional Seas Reports and Studies no. 177 Published by United Nations Environment Programme (UNEP) and the Secretariat of the Convention on the Conservation of Migratory Species of Wild Animals (CMS). Review of Small Cetaceans. Distribution, Behaviour, Migration and Threats. 2004. Compiled for CMS by Boris M. Culik. Illustrations by Maurizio Wurtz, Artescienza. UNEP / CMS Secretariat, Bonn, Germany. 343 pages. Marine Mammal Action Plan / Regional Seas Reports and Studies no. 177 Produced by CMS Secretariat, Bonn, Germany in collaboration with UNEP Coordination team Marco Barbieri, Veronika Lenarz, Laura Meszaros, Hanneke Van Lavieren Editing Rüdiger Strempel Design Karina Waedt The author Boris M. Culik is associate Professor The drawings stem from Prof. Maurizio of Marine Zoology at the Leibnitz Institute of Wurtz, Dept. of Biology at Genova Univer- Marine Sciences at Kiel University (IFM-GEOMAR) sity and illustrator/artist at Artescienza. and works free-lance as a marine biologist. Contact address: Contact address: Prof. Dr. Boris Culik Prof. Maurizio Wurtz F3: Forschung / Fakten / Fantasie Dept. of Biology, Genova University Am Reff 1 Viale Benedetto XV, 5 24226 Heikendorf, Germany 16132 Genova, Italy Email: [email protected] Email: [email protected] www.fh3.de www.artescienza.org © 2004 United Nations Environment Programme (UNEP) / Convention on Migratory Species (CMS). This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. -

Greater Pemba Channel IMMA Description the Pemba Channel Is Located in Northern Tanzania in the Western Indian Ocean
Greater Pemba Channel IMMA Description The Pemba Channel is located in northern Tanzania in the Western Indian Ocean. The channel has steep drop offs in bathymetry and a rapid north flowing current that appears to provide excellent conditions for cetaceans. An assessment of cetaceans along the entire 800km coast of Tanzania showed the Pemba Channel had by far the highest indices of Area Size cetacean diversity and relative abundance in the 2 6,647 km country (Braulik et al. 2017a). Subsequently intensive surveys in the Pemba Channel have Qualifying Species and Criteria confirmed this high diversity; currently 13 cetacean Indo-pacific bottlenose dolphin species are recorded in the cIMMA, however survey Tursiops aduncus effort has been limited and this total is likely to rise Criterion B1 (Braulik et al. 2017b). Many oceanic species have been recorded in the Channel often within sight of Indian Ocean humpback dolphin Sousa plumbea land, including Blainville's beaked whale, Dwarf Criterion A; B1 sperm whale, False killer whale, Short-finned pilot whale, Risso’s dolphin and Fraser’s dolphin. Spinner Marine Mammal Diversity dolphins are extremely common in waters of the Criterion D2 upper shelf, and sometimes occur in groups of over 800 individuals. Other species recorded include Delphinus delphis tropicalis, Globicephala Humpback whales, Pan-tropical spotted dolphin, macrocephalus, Grampus griseus, Kogia breviceps, Common bottlenose dolphin, Indo-pacific Lagenodelphis hosei, Megaptera novaeangliae, bottlenose dolphin and Indian Ocean humpback Mesoplodon densirostris, Pseudorca crassidens, dolphin. Sousa plumbea, Stenella attenuata, Stenella longirostris, Tursiops aduncus, Tursiops truncatus Pemba Island is surrounded by deep water, and Summary there are small, likely resident populations of Indo- pacific bottlenose dolphin and Indian Ocean The Pemba Channel is located in northern humpback dolphins that occur in the shallow Tanzania in the Western Indian Ocean. -

Cetaceans of the Red Sea - CMS Technical Series Publication No
UNEP / CMS Secretariat UN Campus Platz der Vereinten Nationen 1 D-53113 Bonn Germany Tel: (+49) 228 815 24 01 / 02 Fax: (+49) 228 815 24 49 E-mail: [email protected] www.cms.int CETACEANS OF THE RED SEA Cetaceans of the Red Sea - CMS Technical Series Publication No. 33 No. Publication Series Technical Sea - CMS Cetaceans of the Red CMS Technical Series Publication No. 33 UNEP promotes N environmentally sound practices globally and in its own activities. This publication is printed on FSC paper, that is W produced using environmentally friendly practices and is FSC certified. Our distribution policy aims to reduce UNEP‘s carbon footprint. E | Cetaceans of the Red Sea - CMS Technical Series No. 33 MF Cetaceans of the Red Sea - CMS Technical Series No. 33 | 1 Published by the Secretariat of the Convention on the Conservation of Migratory Species of Wild Animals Recommended citation: Notarbartolo di Sciara G., Kerem D., Smeenk C., Rudolph P., Cesario A., Costa M., Elasar M., Feingold D., Fumagalli M., Goffman O., Hadar N., Mebrathu Y.T., Scheinin A. 2017. Cetaceans of the Red Sea. CMS Technical Series 33, 86 p. Prepared by: UNEP/CMS Secretariat Editors: Giuseppe Notarbartolo di Sciara*, Dan Kerem, Peter Rudolph & Chris Smeenk Authors: Amina Cesario1, Marina Costa1, Mia Elasar2, Daphna Feingold2, Maddalena Fumagalli1, 3 Oz Goffman2, 4, Nir Hadar2, Dan Kerem2, 4, Yohannes T. Mebrahtu5, Giuseppe Notarbartolo di Sciara1, Peter Rudolph6, Aviad Scheinin2, 7, Chris Smeenk8 1 Tethys Research Institute, Viale G.B. Gadio 2, 20121 Milano, Italy 2 Israel Marine Mammal Research and Assistance Center (IMMRAC), Mt. -

The Plight of the 'Forgotten' Whales
The Plight of the ‘Forgotten’ Whales It’s mainly smaller cetaceans that are now in peril by Robert L. Brownell, Jr., Katherine Ralls, and William F. Perrin The “Save the Whales” movement, the most moratorium which expires in 1991. successful wildlife crusade in history, has greatly In marked contrast to the improving pros- influenced government policies in a number of pects for the great whales, the status of many countries, including the United States. Thanks in smaller cetaceans has continued to deteriorate large part to the movement’s dedicated mem- over the last two decades. Some species and bers, the fight to save the great whales has been local populations of dolphins, porpoises, and largely won. Yet all but i nored small whales are in greater in this victory has been tie danger of extinction than anv plight of smaller cetaceans, . of thUe great whales, except ‘ which continues to worsen. % Dossiblv the northern rieht The pivotal year for the khale, Eubalaena glaciacs. For great whales was 1970, when example, the population of the nine of the 12 species were baiji, or Chinese River dolphin, listed as endangered under the Lipotes vexillifer, is believed to U.S. Endangered Species Act be down to only about 300 (ESA, box, pp. 12-13). At that individuals. Each year time they met the ESA’s hundreds of thousands of definition of an endangered other small cetaceans are species (Table 1 ). They were killed incidentally in various overexploited by commercial fisheries around the world. whalers and inadequately However, the situation of most protected by laws and of these smaller cetaceans has regulations. -
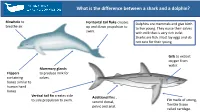
What Is the Difference Between a Shark and a Dolphin?
What is The Difference BetweenWhat a is the difference between a shark and a dolphin? Shark and a Dolphin? Blowhole to Horizontal tail fluke creates Dolphins are mammals and give birth breathe air. up and down propulsion to to live young. They nurse their calves swim. with milk that is very rich in fat. Sharks are fish. Most lay eggs and do not care for their young. Gills to extract oxygen from water. Mammary glands Flippers to produce milk for containing calves. bones similar to human hand bones. Vertical tail fin creates side Additional fins , Fin made of strong, to side propulsion to swim. second dorsal, flexible tissue pelvic and anal. called cartilage. What is The Difference BetweenWhat a is the difference between a whale and a dolphin? Shark and a Dolphin? Two blowholes Baleen Whale Baleen is the bristle like structure to breathe air. in a whale’s upper jaw which it uses to filter small fish or crustaceans from the water. Single blowhole to Many species we call whales breathe air. are more closely related to Throat pleats in some baleen dolphins. Generally scientists whales expand to fill with water Dolphin talk about baleen whales and small fish or crustaceans and and toothed then contract, pushing the water whales. Toothed whales back through the baleen. include sperm whales, beaked whales, and all Teeth on the upper and lower porpoises and dolphins. jaws to grab fish, squid or other Killer whales and pilot prey. They use echolocation to whales are actually dolphins. find their food. What is The Difference BetweenEcholocation a Shark and a Dolphin? Toothed whales (dolphins, porpoises and species like pilot Brain processes Nasal passage whales and killer whales) use echolocation to navigate and find signals to form an contains their food. -

Marine Mammals of Hawai‛I Over 20 Cetaceans (Dolphins and Whales) and One Phocid (Seal) Have Been Observed in Hawaiian Waters
Marine Mammals of Hawai‛i Over 20 cetaceans (dolphins and whales) and one phocid (seal) have been observed in Hawaiian waters. Spinner dolphin Pantropical Spotted dolphin (Stenella longirostris) (Stenella attenuata) Coastal species that feeds at night and Coastal species that feeds at night. Born rests during the day. Often found in large without spots, but gain spots with age. groups of 100+ in div idua ls. We ll known for FdihFound inshore more iflldffhin fall and offshore their acrobatic aerial behaviors. 6-7 feet in more in spring. 6-7 feet in length. length. Bottlenose dolphin False Killer whale (Tursiops truncatus) (Pseudorca crasidens) Inshore and offshore stock. Inshore smaller and Several stocks in Hawaii. Insular population more gray in color. Commonly seen in groups of candidate to be listed endangered under ESA. 2-15 animals, offshore in groups of several Found in groups of 10-20, up to 40 individuals. hundred. Up to 12 feet in length. Prefer deep waters. 15-20 feet in length. Insular population listed as endangered under ESA. Short-finned pilot whale Melon-headed whale (Globicephala macrorhynchus) (Peponocephala electra) Second largest delphinid. Bulbous melon head Prefers deeper waters. Often found in very large with no discernable beak. Found in groups of groups, commonly with over 1000 individuals. 25-50 animals. Form ranks that can be ½ mile Make fast, low leaps as they swim. Approximately in length. 12-18 feet in length, max length = 24 9 feet in length. feet. Hawai‛i’s State Marine Mammal Hawai‛i’s State Mammal Humpback whale Hawaiian Monk seal (Megaptera novaeangliae) (Monachus schauinslandi ) **Federal law prohibits approaching humpback whales within 100 meters One of the rarest marine mammals in the world. -

Physical Habitat of Cetaceans Along the Continental Slope in the North- Central and Western Gulf of Mexico R
MARINE MAMMAL SCIENCE, 14(3):490-507 (July 1998) 0 1998 by the Society for Marine Mammalogy PHYSICAL HABITAT OF CETACEANS ALONG THE CONTINENTAL SLOPE IN THE NORTH- CENTRAL AND WESTERN GULF OF MEXICO R. W. DAVIS G. S. FARGION' Texas Institute of Oceanography, Texas A&M University at Galveston, Galveston, Texas 77553, U.S.A. E-mail: [email protected] N. MAY T. D. LEMING M. BAUMGARTNER Southeast Fisheries Science Center, National Marine Fisheries Service, NOAA, Building 1103, Room 218, Stennis Space Center, Mississippi 39529, U.S.A. W. E. EVANS Texas Institute of Oceanography, Texas A&M University at Galveston, Galveston, Texas 77553, U.S.A. L. J. HANSEN Southeast Fisheries Science Center, National Marine Fisheries Service, NOAA, 2 17 Fort Johnson Road, Charleston, South Carolina 29412, U.S.A. IS. MULLIN Southeast Fisheries Science Center, National Marine Fisheries Service, NOAA, P.O. Drawer 1207, Pascagoula, Mississippi 39568, U.S.A. ABSTRACT The physical habitat of cetaceans found along the continental slope in the north-central and western Gulf of Mexico was characterized from shipboard sighting data, simultaneous hydrographic measurements, and satellite remote sensing. The study area was encompassed by the longitude of the Florida- Alabama border (87.5"W), the southernmost latitude of the Texas-Mexico Current affiliation: Data formation LLC, Bethesda, Maryland 20816, U.S.A. 490 DAVIS ET AL.: CETACEANS OF THE GULF OF MEXICO 49 1 border (26.0°N), and the 100-m and 2,000-m isobaths. Shipboard surveys were conducted seasonally for two years from April 1992 to May 1994.