False Positive Morphologic Diagnoses at the Anomaly Scan: Marginal Or
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Trimester Anomaly Scan Using Virtual Reality (VR FETUS Study): Study Protocol for a Randomized Clinical Trial C
Pietersma et al. BMC Pregnancy and Childbirth (2020) 20:515 https://doi.org/10.1186/s12884-020-03180-8 STUDY PROTOCOL Open Access First trimester anomaly scan using virtual reality (VR FETUS study): study protocol for a randomized clinical trial C. S. Pietersma1, A. G. M. G. J. Mulders1, L. M. Moolenaar1, M. G. M. Hunink2,3,4, A. H. J. Koning5, S. P. Willemsen6, A. T. J. I. Go1, E. A. P. Steegers1 and M. Rousian1* Abstract Background: In recent years it has become clear that fetal anomalies can already be detected at the end of the first trimester of pregnancy by two-dimensional (2D) ultrasound. This is why increasingly in developed countries the first trimester anomaly scan is being offered as part of standard care. We have developed a Virtual Reality (VR) approach to improve the diagnostic abilities of 2D ultrasound. Three-dimensional (3D) ultrasound datasets are used in VR assessment, enabling real depth perception and unique interaction. The aim of this study is to investigate whether first trimester 3D VR ultrasound is of additional value in terms of diagnostic accuracy for the detection of fetal anomalies. Health-related quality of life, cost-effectiveness and also the perspective of both patient and ultrasonographer on the 3D VR modality will be studied. Methods: Women in the first trimester of a high risk pregnancy for a fetus with a congenital anomaly are eligible for inclusion. This is a randomized controlled trial with two intervention arms. The control group receives ‘care as usual’: a second trimester 2D advanced ultrasound examination. The intervention group will undergo an additional first trimester 2D and 3D VR ultrasound examination. -

GMEC) Strategic Clinical Networks Reduced Fetal Movement (RFM
Greater Manchester & Eastern Cheshire (GMEC) Strategic Clinical Networks Reduced Fetal Movement (RFM) in Pregnancy Guidelines March 2019 Version 1.3a GMEC RFM Guideline FINAL V1.3a 130619 Issue Date 15/02/2019 Version V1.3a Status Final Review Date Page 1 of 19 Document Control Ownership Role Department Contact Project Clinical Lead Manchester Academic Health [email protected] Science Centre, Division of Developmental Biology and Medicine Faculty of Biology, Medicine and Health, The University of Manchester. Project Manager GMEC SCN [email protected] Project Officer GMEC SCN [email protected] Endorsement Process Date of Presented for ratification at GMEC SCN Maternity Steering Group on:15th February ratification 2019 Application All Staff Circulation Issue Date: March 2019 Circulated by [email protected] Review Review Date: March 2021 Responsibility of: GMEC Maternity SCN Date placed on March 2019 the Intranet: Acknowledgements On behalf of the Greater Manchester and Eastern Cheshire and Strategic Clinical Networks, I would like to take this opportunity to thank the contributors for their enthusiasm, motivation and dedication in the development of these guidelines. Miss Karen Bancroft Maternity Clinical Lead for the Greater Manchester & Eastern Cheshire SCN GMEC RFM Guideline FINAL V1.3a 130619 Issue Date 15/02/2019 Version V1.3a Status Final Review Date Page 2 of 19 Contents 1 What is this Guideline for and Who should use it? ......................................................................... 4 2 What -

ANC Level 1 2Nd Edition.Pdf
Pregnancy & Childbirth Management Guidelines Level- 1 A Guide for Nurses, Midwives and Doctors Second Edition DEPARTMENT OF WOMAN &CHILD HEALTH DIRECTORATE GENERAL OF PRIMARY HEALTH CARE MINISTRY OF HEALTH SULTANATE OF OMAN 2016 ML- 60 Pregnancy & Childbirth Management Guidelines Level- 1 A Guide for Nurses, Midwives and Doctors Second Edition 2016 I II ACKNOWLEDGEMENT Acknowledgement with gratitude to all contributors & Reviewers for their effort in updating this guideline manual: Contributors from Woman & Child Health Department: • Dr. Jamila Al-Abri, Senior Specialist, Dept. of Woman & Child Health • Dr. Fatima Al Hinai, Senior Specialist, Director of Woman & Child Health Dept. • Dr. Nawal Al Rashdi, Senior Specialist, Dept. of Woman & Child Health • Dr. Salwa Jabbar Alshahabi, Specialist, Dept. of Woman & Child Health • Dr. Omaima Abdel Wahab, Senior Medical Officer, Dept. of Woman & Child Health. Contributors from Primary Health Care Institutions: • Dr. Nabila Al Wahaibi, Senior Consultant (FAMCO), Wadi Kabeer Health Centre. • Dr. Ahdab Abdul Hafeez, Specialist, Muscat Health Centre. • Dr. Imrana Masoud, Senior Medical Officer, Al Seeb Health Centre. Contributors from National Diabetic & Endocrine Centre & NCD department • Dr. Noor Al Busaidi, Senior Consultant, Director of National Diabetic & Endocrine Centre (NDEC) • Dr. Hilal Al Musailhi, Senior Consultant Adult Endocrinology, (NDEC). • Dr. Deepa Manoharan , Medical Officer, (NDEC). • Dr. Nada Hareb Al Sumri, Senior Specialist, Non Communicable Disease Dept • Dr. Suleiman Al Shereigi, Senior Specialist in Public Health Administration,(NDEC) Reviewers from Secondary & Tertiary Heath Care : • Dr. Tamima Al Dughaishi, Senior Consultant Obstetrics & Gynecology, SQUH • Dr. Bernadette Punnoose, Senior Consultant Obstetrics & Gynecology, Royal Hospital • Dr. Badrya Al Fahdi, Senior Consultant Obstetrics & Gynecology, Royal Hospital • Dr. Sumaya Al Amri, Senior Specialist Obstetrics & Gynecology, Royal Hospital • Dr. -

Prenatal Ultrasonography of Craniofacial Abnormalities
Prenatal ultrasonography of craniofacial abnormalities Annisa Shui Lam Mak, Kwok Yin Leung Department of Obstetrics and Gynaecology, Queen Elizabeth Hospital, Hong Kong SAR, China REVIEW ARTICLE https://doi.org/10.14366/usg.18031 pISSN: 2288-5919 • eISSN: 2288-5943 Ultrasonography 2019;38:13-24 Craniofacial abnormalities are common. It is important to examine the fetal face and skull during prenatal ultrasound examinations because abnormalities of these structures may indicate the presence of other, more subtle anomalies, syndromes, chromosomal abnormalities, or even rarer conditions, such as infections or metabolic disorders. The prenatal diagnosis of craniofacial abnormalities remains difficult, especially in the first trimester. A systematic approach to the fetal Received: May 29, 2018 skull and face can increase the detection rate. When an abnormality is found, it is important Revised: June 30, 2018 to perform a detailed scan to determine its severity and search for additional abnormalities. Accepted: July 3, 2018 Correspondence to: The use of 3-/4-dimensional ultrasound may be useful in the assessment of cleft palate and Kwok Yin Leung, MBBS, MD, FRCOG, craniosynostosis. Fetal magnetic resonance imaging can facilitate the evaluation of the palate, Cert HKCOG (MFM), Department of micrognathia, cranial sutures, brain, and other fetal structures. Invasive prenatal diagnostic Obstetrics and Gynaecology, Queen Elizabeth Hospital, Gascoigne Road, techniques are indicated to exclude chromosomal abnormalities. Molecular analysis for some Kowloon, Hong Kong SAR, China syndromes is feasible if the family history is suggestive. Tel. +852-3506 6398 Fax. +852-2384 5834 E-mail: [email protected] Keywords: Craniofacial; Prenatal; Ultrasound; Three-dimensional ultrasonography; Fetal structural abnormalities This is an Open Access article distributed under the Introduction terms of the Creative Commons Attribution Non- Commercial License (http://creativecommons.org/ licenses/by-nc/3.0/) which permits unrestricted non- Craniofacial abnormalities are common. -

Fetal Medicine August 2010
Advanced Training Skills Module – Fetal Medicine August 2010 Fetal Medicine This module is designed to prepare the future consultant for dealing with congenital abnormalities detected during pregnancy. This includes the organisation and supervision of screening programmes for structural and chromosomal anomalies. Many of these cases need to be managed within a multidisciplinary team which includes clinical geneticists and fetal medicine subspecialists. Apart from a sound knowledge of embryology and fetal physiology, clinicians working in this field must be competent in the prenatal diagnosis of common abnormalities. They also require a sound working knowledge of clinical and laboratory genetics in order that they can investigate and, where appropriate, refer suitable families. Competence in obstetric ultrasound is a prerequisite for advanced skills in prenatal diagnosis and fetal medicine. Trainees must complete the new Intermediate Ultrasound of Fetal Anatomy module prior to entry into the ATSM in Fetal Medicine. Attendance at a suitable Fetal Medicine theoretical course is a compulsory requirement of the module. This must be attended before completion of the ATSM and can have been done not more than three years previously. Specifically, once trained, individuals should: Work well as part of a multidisciplinary team Understand the organization of prenatal screening and diagnostic services at a local and regional level Be clinically competent in the prenatal diagnosis, counselling and management of common fetal abnormalities and markers of chromosomal abnormality. Be clinically competent and certified in first trimester screening for chromosomal abnormality by a combination of nuchal translucency assessment and biochemical marker assays. Be clinically competent at amniocentesis and have a sound knowledge of the principles and techniques of first trimester chorion villus biopsy. -

Chapter III: Case Definition
NBDPN Guidelines for Conducting Birth Defects Surveillance rev. 06/04 Appendix 3.5 Case Inclusion Guidance for Potentially Zika-related Birth Defects Appendix 3.5 A3.5-1 Case Definition NBDPN Guidelines for Conducting Birth Defects Surveillance rev. 06/04 Appendix 3.5 Case Inclusion Guidance for Potentially Zika-related Birth Defects Contents Background ................................................................................................................................................. 1 Brain Abnormalities with and without Microcephaly ............................................................................. 2 Microcephaly ............................................................................................................................................................ 2 Intracranial Calcifications ......................................................................................................................................... 5 Cerebral / Cortical Atrophy ....................................................................................................................................... 7 Abnormal Cortical Gyral Patterns ............................................................................................................................. 9 Corpus Callosum Abnormalities ............................................................................................................................. 11 Cerebellar abnormalities ........................................................................................................................................ -

Prenatal Prediction of Outcome by Fetal Gastroschisis in a Tertiary Referral Center
diagnostics Article Prenatal Prediction of Outcome by Fetal Gastroschisis in a Tertiary Referral Center Katharina Nitzsche 1, Guido Fitze 2, Mario Rüdiger 3 and Cahit Birdir 1,* 1 Department of Obstetrics and Gynecology, University Clinic of Carl Gustav Carus Dresden, Technische Universität Dresden, 01307 Dresden, Germany; [email protected] 2 Department of Pediatric Surgery, University Clinic of Carl Gustav Carus Dresden, Technische Universität Dresden, 01307 Dresden, Germany; guido.fi[email protected] 3 Department of Pediatrics, University Clinic of Carl Gustav Carus Dresden, Technische Universität Dresden, 01307 Dresden, Germany; [email protected] * Correspondence: [email protected] Received: 4 June 2020; Accepted: 30 July 2020; Published: 30 July 2020 Abstract: The aim of this study was to find a prenatal parameter to be able to predict possible prenatal complications or postnatal surgical options, thus allowing the fetal medicine specialist, together with pediatric surgeons and neonatologists, to improve the counseling of the parents and to determine the timing of delivery and therapy. This was a retrospective analysis of prenatal diagnosis and outcome of fetuses with 34 cases of gastroschisis between the years 2007 and 2017. A total of 34 fetuses with gastroschisis were examined and 33 outcomes registered: 22 cases of simple gastroschisis (66.7%) and 11 cases of complex gastroschisis (33.3%). A cut-off value of 18 mm for intraabdominal bowel dilatation (IABD) showed a positive predictive value (PPV) of 100% for predicting simple gastroschisis. IABD gives the best prediction for simple versus complex gastroschisis (cut-off of 18 mm). Extra-abdominal bowel dilatation (EABD) cut-off values of 10 mm and 18 mm showed low sensitivity and specificity to predict complex gastroschisis. -

MATERNAL SCREENING for FOETAL ABNORMALITY Assessment Assessment
REP ORT MATERNAL SCREENING FOR FOETAL ABNORMALITY assessment assessment HEALTH TECHNOLOGY ASSESSMENT UNIT MEDICAL DEVELOPMENT DIVISION health MINISTRY OF HEALTH MALAYSIA MOH/PAK/59.03(TR) 1 MEMBERS OF EXPERT COMMITTEE Dr Zaridah Shafie Obstetric & Gynecology Consultant Kangar Hospital Dr Zulkfili Mohd Kassim Pakar Perunding O & G Hospital Kuala Terengganu Dr Mohd Rouse Abd Majid Obstetric & Gynecology Consultant Sg Petani Hospital Dr Neoh Siew Hong Pediatric Consultant Taiping Hospital Dr Rosnah Sutan Jabatan Kesihatan Bersekutu National University of Malaysia Prof Jamiyah Hassan Faculty of Medicine University Malaya Project Coordinators Dr S Sivalal Deputy Director Health Technology Assessment Unit Ministry of Health Malaysia Dr Rusilawati Jaudin Principal Assistant Director Health Technology Assessment Unit Ministry of Health Malaysia Ms Sin Lian Thye Nursing Sister Health Technology Assessment Unit Ministry of Health Malaysia 2 EXECUTIVE SUMMARY INTRODUCTION Congenital malformations are structural or anatomical defects that are present at birth, resulting from influences acting on the developing embryo in early pregnancy. Some congenital malformations are potentially preventable; however, they remain major causes of early death, hospitalization of infants and young children and significant long-term physical and developmental disabilities. Screening and early detection of Downs Syndrome and other chromosomal anomalies in-utero provides several benefits like the opportunity to inform parents and counseling on the likelihood of delivery -
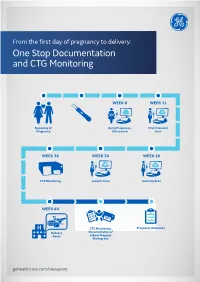
One Stop Documentation and CTG Monitoring
From the first day of pregnancy to delivery: One Stop Documentation and CTG Monitoring WEEK 8 WEEK 11 Beginning of Early Pregnancy First Trimester Pregnancy Ultrasound Scan WEEK 36 WEEK 30 WEEK 18 CTG Monitoring Growth Scan Anomaly Scan WEEK 40 CTG Monitoring, Pregnancy Outcomes Delivery Documentation of Room Labour Progress (Partogram) gehealthcare.com/viewpoint From the first ultrasound examination to delivery, ViewPoint™ 6 in combination with Trium CTG Online1 can help you focus on the patient and her baby. Your clinical workflows in the OB/GYN department can now be covered by a single solution. GE offers you a comprehensive solution for digital, paperless documentation throughout pregnancy and birth. ViewPoint 6 was developed to significantly simplify image management, reporting and workflows in hospitals and private practices. Patient data, detailed and structured findings, and images are clearly available in one view. ViewPoint 6 offers examination types for: • Pelvic Ultrasound (including IOTA2) • Growth Scan • Early Pregnancy • Fetal Wellbeing including Biophysical Profile • 1st Trimester Ultrasound including FMF risk assessment2 • Pregnancy Outcome • 2nd/3rd Trimester Ultrasound ViewPoint 6 can be linked to Trium CTG Online1. Trium CTG Online allows you to keep track of all CTG traces, provides decision support and digital archiving and exchange of delivery data for documentation purposes. Trium CTG Online allows to keep your focus on the patient Centralized and Decentralized CTG Monitoring Comprehensive Documentation • Survey -

Guidelines for Professional Ultrasound
Guidelines For Professional Ultrasound Society and College of Radiographers and British Medical Ultrasound Society Revision 3, December 2018 SCoR/BMUS Guidelines for Professional Ultrasound Practice. Revision 3, December 2018 Minor amendments, March 2019. SOCIETY AND COLLEGE OF RADIOGRAPHERS AND BRITISH MEDICAL ULTRASOUND SOCIETY GUIDELINES FOR PROFESSIONAL ULTRASOUND PRACTICE DECEMBER 2015 Revision 3, December 2018. Minor amendments, March 2019 Contents Acknowledgements ...................................................................................................................................................... 4 Foreword ...................................................................................................................................................................... 6 December 2015 edition ........................................................................................................................................ 6 Revision 1, December 2016 .................................................................................................................................. 6 Revision 2, December 2017 .................................................................................................................................. 7 Revision 3, December 2018. Minor post publication amends March 2019 ....................................................... 7 SECTION 1: GENERAL INFORMATION ........................................................................................................................ -

Women's Health and Wellbeing
INNERMOSTT HE LTHCARE PRIVATE MEDICAL CLINIC Women’s Health and Wellbeing Safe in our hands www.innermosthealthcare.com Welcome from Dr Bryan Beattie Welcome to Innermost Healthcare, the leading private women’s healthcare clinic in Wales. Based at our luxuriously many other scanning only appointed, state-of-the-art companies, you have clinic in Cardiff, we provide immediate access to Dr the very latest in pregnancy Bryan Beattie, Consultant scanning technology and in Fetal Medicine, should “ The extra reassurance expert advice from specialist problems arise. and peace of mind Dr clinicians in genetics and We hope you find this Beattie gave us was maternity care, aesthetics and overview of our services general women’s healthcare helpful and would be worth every penny services. We are passionate delighted to discuss how we ” about delivering the highest can support you whether quality care for women in you are trying to conceive, Wales and the wider UK. currently pregnant or just We believe that a visit to need women’s healthcare. our clinic gives you and your baby the very best of care, by Dr Bryan Beattie providing you with unrivalled MD FRCOG levels of personalised support, Founder and CEO, with access to many scans Innermost Healthcare and tests not available on Chairman of Innermost the NHS at this time. Unlike Learning Charity 2 Innermost Healthcare About us Dr Bryan Beattie MD FRCOG is a Consultant Obstetrician and an accredited Specialist Consultant in Fetal and Maternal Medicine, based at the University Hospital of Wales in Cardiff. In 1995, he was responsible for setting up their Early Pregnancy Assessment Unit. -

Second Trimester Anomaly Scan Using 3D/4D Ultrasound
DSJUOG Fernando Bonilla-Musoles et al 10.5005/jp-journals-10009-1424 REVIEW ARTICLE Second Trimester Anomaly Scan using 3D/4D Ultrasound 1Fernando Bonilla-Musoles, 2Francisco Bonilla Jr, 3Francisco Raga, 4Oscar Caballero 5Clodoaldo Cadete, 6Luiz Eduardo Machado ABSTRACT ultrasound, such as the 3D/4D and their modes (STIC: The use of three-dimensional/four-dimensional (3D/4D) spatial temporal image correlation; AVC: Automatic ultrasound has become 'universalʼ in the increasingly precise volume calculation; VOCAL: Virtual organ computer- diagnosis of fetal malformations. The introduction of new aided analysis and the inverse mode), more recently the ultrasound modes, such as the HDlive or the Radiance HDlive and Radiance System Architecture (RSA). In System Architecture (RSA), which improve even more the this chapter, we are going to show normal images and quality of images, makes it easier to examine normal embryos and fetuses with incredible perfection and achieve diagnosis curious cases of malformations that have been observed of malformations, increasingly complex and of high clinical recently with the use of 3D/4D, HDlive and RAS. They importance. can demonstrate that these new technologies are Keywords: Three-dimensional/four-dimensional ultrasound, really useful. Fetal malformations, HDlive, Radiance system architecture, Referring to the 3D/4D bibliography, we have only Second trimester. showed quotations by some authors; among us, the most 1-32 How to cite this article: Bonilla-Musoles F, Bonilla F Jr, Raga F, published ones in this field. Concerning the HDlive in Caballero O, Cadete C, Machado LE. Second Trimester Anomaly obstetrics, the literature, even being poor,33-61 is enough Scan using 3D/4D Ultrasound.