Modeling the Effects of Sleep Debt: Actively Promotes Wakefulness More So Than Sleep
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sleep Matters the Impact of Sleep on Health and Wellbeing Mental Health Awareness Week 2011
Sleep Matters The impact of sleep on health and wellbeing Mental Health Awareness Week 2011 Address Mental Health Foundation Sea Containers House 20 Upper Ground London SE1 9QB United Kingdom Telephone 020 7803 1100 Email [email protected] Website www.HowDidYouSleep.org £10 IBSN 978-1-906162-65-8 Registered charity number England 801130 Scotland SC039714 © Mental Health Foundation 2011 Contents 04 Executive summary 08 Introduction 12 Part 01 – Sleeping and sleep patterns 28 Part 02 – Poor sleep 48 Part 03 – Sleeping well 62 Conclusion 66 Useful resources 68 References 72 Appendix: Sleep diary 76 Acknowledgements 01 ‘The main facts in human life are five: E. M. Forster Executive We spend approximately a Poor sleep over a sustained period One of the most widely used and – The new Public Health Outcomes third of our lives asleep. Sleep leads to a number of problems which successful therapies is Cognitive Framework should include a specific Summary are immediately recognisable, including Behavioural Therapy (CBT). This is outcome on reducing sleep problems is an essential and involuntary fatigue, sleepiness, poor concentration, useful even for people who have across the whole population. process, without which we lapses in memory, and irritability. had insomnia for a long period of time. Sleep should also be reflected in cannot function effectively. A full course of such a therapy with new national mental health outcome It is as important to our Up to one third of the population may a sleep specialist is potentially costly, indicators, including improving bodies as eating, drinking suffer from insomnia (lack of sleep and is most appropriate for people sleep for people who experience and breathing, and is vital for or poor quality sleep). -

Slow-Wave Sleep, Diabetes, and the Sympathetic Nervous System
COMMENTARY Slow-wave sleep, diabetes, and the sympathetic nervous system Derk-Jan Dijk* Surrey Sleep Research Centre, Faculty of Health and Medical Sciences, University of Surrey, Guildford, Surrey GU2 7XP, United Kingdom leep oscillates between two dif- of SWS that has accumulated. The latter less provides supportive evidence for the ferent states: non-rapid eye conclusion was derived from SWS depri- notion that SWS is restorative also for movement (NREM) sleep and vation experiments in which stimuli, usu- the body and that negative effects asso- rapid-eye movement (REM) ally acoustic stimuli [although early on ciated with disruption of this state may Ssleep. Slow-wave sleep (SWS) is a sub- in the history of SWS deprivation, mild extend to the body. state of NREM sleep, and its identifica- electric shocks were used (5)], are deliv- Many other physiological variables are tion is based primarily on the presence ered in response to the ongoing EEG. affected by the behavioral-state sleep, of slow waves, i.e., low-frequency, high- The drive to enter SWS is strong and is the NREM–REM cycle, and SWS. amplitude oscillations in the EEG. Upon the transition from wakefulness to Quantification of SWS is accomplished sleep, heart rate slows down. During by visual inspection of EEG records or Short habitual sleep sleep, the balance of sympathetic and computerized methods such as spectral parasympathetic tone oscillates in syn- analysis based on the fast Fourier trans- has been associated chrony with the NREM–REM cycle. form (FFT). Slow-wave activity (SWA; Analysis of autonomic control of the also referred to as delta power) is a with increased risk variability of heart rate demonstrates quantitative measure of the contribution that, within each NREM episode, as of both the amplitude and prevalence of for diabetes. -

Sleep 101: the Abcs of Getting Your Zzzs
Sleep 101: The ABCs of Getting Your ZZZs Steven D. Brass, MD MPH MBA Director of Neurology Sleep Medicine Clinic UC Davis Health System November 18, 2014 What you will learn: • Why do we sleep? • How much sleep do we need? • What are the effects of sleep deprivation? • What are the different stages of sleep? • What are the types of sleep problems? • What is sleep apnea and how is it treated? • How can we sleep better? Why do we sleep? • Each of us will spend about 1/3 of our lifetime sleeping! • Sleep helps us with: – Memory consolidation – Immune system – Recharge energy for the day – Growth and development How much sleep do we need? Infants : 14-15 hours National Sleep Foundation Secrets of Sleep; National Geographic Magazine . 2010 Adolescents: 8.5-9.25 hours National Sleep Foundation Secrets of Sleep; National Geographic Magazine . 2010 Adult/Elder Sleep: 7-9 hours National Sleep Foundation Secrets of Sleep; National Geographic Magazine . 2010 What are the different stages of sleep? • Non REM Sleep -75% of the night – Stage 1 – Stage 2 – Stage 3 – Stage 4 • REM Sleep -25% of the night – Dreaming Normal Sleep Patterns in Young Adults REM Stage AWAKE NREM REM 1 2 3 4 1 2 3 4 5 6 7 8 Hours of Sleep Adapted from Berger RJ. The sleep and dream cycle. In: Kales A, ed. Sleep Physiology & Pathology: A Symposium. Philadelphia: J.B. Lippincott; 1969. American Academy of Sleep Medicine Sleep Fragmentation Affects Sleep Quality NORMAL SLEEP = Paged ON CALL SLEEP © American Academy of Sleep Medicine, Westchester, IL Why do we dream? • Everyone -

Sleep Disorders Preeti Devnani
SPECIAL ISSUE 1: INVITED ARTICLE Sleep Disorders Preeti Devnani ABSTRACT Sleep disorders are an increasingly important and relevant burden faced by society, impacting at the individual, community and global level. Varied presentations and lack of awareness can make accurate and timely diagnosis a challenge. Early recognition and appropriate intervention are a priority. The key characteristics, clinical presentations and management strategies of common sleep disorders such as circadian rhythm disorders, restless legs syndrome, REM behavior disorder, hypersomnia and insomnia are outlined in this review. Keywords: Hypersomnia, Insomnia, REM behavior International Journal of Head and Neck Surgery (2019): 10.5005/jp-journals-10001-1362 INTRODUCTION Department of Neurology and Sleep Disorder, Cleveland Clinic, Abu Sleep disorders are becoming increasingly common in this modern Dhabi, United Arab Emirates era, resulting from several lifestyle changes. These complaints may Corresponding Author: Preeti Devnani, Department of Neurology present excessive daytime sleepiness, lack of sleep or impaired and Sleep Disorder, Cleveland Clinic, Abu Dhabi, United Arab Emirates, quality, sleep related breathing disorders, circadian rhythm disorder e-mail: [email protected] misalignment and abnormal sleep-related movement disorders.1 How to cite this article: Devnani P. Sleep Disorders. Int J Head Neck They are associated with impaired daytime functioning, Surg 2019;10(1):4–8. increased risk of cardiovascular and cerebrovascular disease, poor Source of support: Nil glycemic control, risk of cognitive decline and impaired immunity Conflict of interest: None impacting overall morbidity and mortality. Diagnosis of sleep disorders is clinical in many scenarios, The following circadian rhythm sleep–wake disorders adapted polysomnography is a gold standard for further evaluation of from the ICSD-3: intrinsic sleep disorder such as obstructive sleep apnea (OSA) • Delayed sleep–wake phase disorder and periodic limb movement disorder (PLMD). -

Ac 120-100 06/07/10
U.S. Department Advisory of Transportation Federal Aviation Administration Circular Date: 06/07/10 AC No: 120-100 Subject: Basics of Aviation Fatigue Initiated by: AFS-200 Change: 1. PURPOSE. This advisory circular (AC): • Summarizes the content of the FAA international symposium on fatigue, “Aviation Fatigue Management Symposium: Partnerships for Solutions”, June 17-19, 2008; • Describes fundamental concepts of human cognitive fatigue and how it relates to safe performance of duties by employees in the aviation industry; • Provides information on conditions that contribute to cognitive fatigue; and • Provides information on how individuals and aviation service providers can reduce fatigue and/or mitigate the effects of fatigue. 2. APPLICABILITY. This AC is not mandatory and does not constitute a regulation. 3. DEFINITIONS. a. Circadian Challenge. Circadian challenge refers to the difficulty of operating in opposition to an individual’s normal circadian rhythms or internal biological clock. This occurs when the internal biological clock and the sleep/wake cycle do not match the local time. For example, the sleep period is occurring at an adverse circadian phase when the body wants to be awake. Engaging in activities that are opposite of this natural biological system represents the circadian challenge (e.g., night work, shift work, jet lag). b. Cognitive Performance. Cognitive performance refers to the ability to process thought and engage in conscious intellectual activity, e.g., reaction times, problem solving, vigilant attention, memory, cognitive throughput. Various studies have demonstrated the negative effects of sleep loss on cognitive performance. c. Circadian Rhythm. A circadian rhythm is a daily alteration in a person’s behavior and physiology controlled by an internal biological clock located in the brain. -

Sleep Manual
Sleep and Recovery An applicable approach to a lifestyle of recovery and rest for athletes 2 TABLE OF CONTENTS INTRODUCTION: WHY SLEEP?.................................................................................. 4 SLEEP AS A PREDICTOR OF PERFORMANCE............................................................ 5 EVIDENCE TO SUPPORT THE THEORY...................................................................... 7 WITHOUT SLEEP......................................................................................................... 8 HORMONES, SLEEP, AND RECOVERY....................................................................... 9 SLEEP IS TRAINING TOO! ........................................................................................ 10 THE IMPORTANCE OF TIMING................................................................................ 11 REM SLEEP................................................................................................................. 12 SOCIAL DRUGS AND SLEEP..................................................................................... 12 SLEEP NUTRITION.................................................................................................... 14 STIMULANTS AND DISTURBANCES TO SLEEP....................................................... 15 TECHNOLOGY AND SLEEP....................................................................................... 17 "STUDENT" ATHLETE............................................................................................... 19 SLEEP AND SOCIAL -
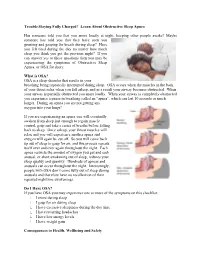
Learn About Obstructive Sleep Apnea
Trouble Staying Fully Charged? Learn About Obstructive Sleep Apnea Has someone told you that you snore loudly at night, keeping other people awake? Maybe someone has told you that they have seen you grunting and gasping for breath during sleep? Have you felt tired during the day no matter how much sleep you think you got the previous night? If you can answer yes to these questions then you may be experiencing the symptoms of Obstructive Sleep Apnea, or OSA for short. What is OSA? OSA is a sleep disorder that results in your breathing being repeatedly interrupted during sleep. OSA occurs when the muscles in the back of your throat relax when you fall asleep, and as a result your airway becomes obstructed. When your airway is partially obstructed you snore loudly. When your airway is completely obstructed you experience a pause in breathing called an “apnea”, which can last 10 seconds or much longer. During an apnea you are not getting any oxygen into your lungs! If you are experiencing an apnea you will eventually awaken from sleep just enough to regain muscle control, gasp and take a series of breaths before falling back to sleep. Once asleep, your throat muscles will relax and you will experience another apnea and oxygen will again be cut off. So you will come back up out of sleep to gasp for air, and this process repeats itself over and over again throughout the night. Each apnea restricts the amount of oxygen you get and each arousal, or short awakening out of sleep, reduces your sleep quality and quantity. -

Shift-Work Disorder
SUPPLEMENT TO Support for the publication of this supplement was provided by Cephalon, Inc. It has been edited and peer reviewed by The Journal of Family Practice. Available at jfponline.com VOL 59, NO 1 / JANUARY 2010 Shift-work disorder The social and economic burden of shift-work disorder } Larry Culpepper, MD, MPH The characterization and pathology of circadian rhythm sleep disorders } Christopher L. Drake, PhD Recognition of shift-work disorder in primary care } Jonathan R. L. Schwartz, MD Managing the patient with shift-work disorder } Michael J. Thorpy, MD Disclosures Dr Culpepper reports that he serves as a con- sultant to AstraZeneca, Eli Lilly and Company, Shift-work disorder Pfizer Inc, Wyeth, sanofi-aventis, and Takeda Pharmaceuticals North America, Inc, and on the speakers bureau of Wyeth. CONTENts AND FACUltY Dr Drake reports that he has received research support from Cephalon, Inc., Takeda Pharma- ceuticals North America, Inc, and Zeo, Inc., and has served on the speakers bureaus of The social and economic Cephalon, Inc., and as a consultant to sanofi- aventis. burden of shift-work disorder ......................... S3 Dr Schwartz reports that he serves as a Larry Culpepper, MD, MPH consultant to and on the speakers bureaus of Department of Family Medicine AstraZeneca, Boehringer Ingelheim Phar- Boston University Medical Center maceuticals, Inc., Cephalon, Inc., Pfizer Inc, Boston, Massachusetts Sepracor Inc., Takeda Pharmaceuticals North America, Inc, and GlaxoSmithKline. Dr Thorpy reports that he serves as a The characterization and pathology consultant to and on the speakers bureaus of Cephalon, Inc., and Jazz Pharmaceuticals, Inc. of circadian rhythm sleep disorders ......... S12 Christopher L. -

Stanford Center for Sleep Sciences and Medicine
Stanford Center for Sleep Sciences and Medicine MEDICINE FACULTY SPOTLIGHTS The pace of our lives has never been more intense. It seems our society has become permanently wired into the “on” position, with no dimmer switch in sight. We’re expected to keep up with the latest news, care for our families, stay connected with friends, and remain available to our employers. In this world of hyper-stress and information overload, sleep has become a precious and scarce commodity. Sleep disorders impact more than 60 percent of adults at some point during their lifetime. The price we pay for our lack of sleep is high: it affects the mind as well as the body. Chronic sleep deprivation has enormous medical consequences for cardiovascular, metabolic, and psychiatric conditions. Sleep and circadian rhythm Emmanuel Mignot, MD, PhD dysfunction are associated with pain and allergies, as well as neurodegener- An eminent sleep scientist who has trans- ative and neuromuscular disorders. formed our understanding of narcolepsy Stanford is recognized by the world as the birthplace of sleep medicine and and its origins, Dr. Mignot and his colleagues were the first to discover that narcolepsy is a beacon in the field of sleep research. We coined the term “obstructive sleep an autoimmune disease caused by loss of apnea,” established gold-standard clinical evaluations such as the multiple hypocretin. Dr. Mignot created a database sleep latency test, and discovered the cause of narcolepsy. Our researchers made up of data from thousands of patients, are continuing to uncover sleep’s hidden mysteries and are setting the highest providing an invaluable resource for study standards of care globally. -

ERS School Course Sleep Medicine: Non-Respiratory Causes of Excessive Daytime Sleepiness
breathe reviews.qxd 01/03/2006 18:09 Page 29 REVIEW The ERS designates this ERS School Course educational activity for a maximum of 1 CME credit. For Sleep Medicine information on how to earn CME credits, see page 292. Non-respiratory causes of excessive daytime sleepiness V. Viot-Blanc Unité de Sommeil Hôpital Lariboisière 2 rue Ambroise Pare 75010 Paris France Fax: 33 149958313 E-mail: [email protected] Educational aims k To present the symptoms, methods of assessment and the different causes of excessive day- time sleepiness when nocturnal respiratory disturbances have been excluded. k To enable physicians to confirm a diagnosis of sleepiness and recognise the pathology. Summary Excessive daytime sleepiness (EDS) is defined as an inability to stay awake and alert dur- ing the major waking episodes of the day, resulting in unintended drowsiness or sleep episodes almost daily for at least 3 months. This review will examine the assessment of sleepiness and different causes of EDS. factors (time of day) and sleep debt (sleep Physiopathology duration and fragmentation). Wake propensity Sleepiness is the result of the combined effects depends on external (environment, exercise, of sleep propensity and wake propensity (fig- drugs, etc.) and internal factors, such as the diff- ure 1). Sleep propensity depends on circadian erent types of hypersomnia or other medical Breathe | March 2006 | Volume 2 | No 3 245 breathe reviews.qxd 01/03/2006 18:09 Page 30 REVIEW ERS School Course Figure 1 Schematic examining the causes Sleep debt of sleepiness. Sleep fragmentation Internal factors Environment Internal clock Trait (?) Sleep propensity Wake propensity Sleepiness disorders usually responsible for EDS. -

CSF Hypocretin-1 Levels in Narcolepsy, Kleine-Levin Syndrome
1667 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.12.1667 on 24 November 2003. Downloaded from PAPER CSF hypocretin-1 levels in narcolepsy, Kleine-Levin syndrome, and other hypersomnias and neurological conditions Y Dauvilliers, CR Baumann, B Carlander, M Bischof, T Blatter, M Lecendreux, F Maly, A Besset, J Touchon, M Billiard, M Tafti, CL Bassetti ............................................................................................................................... J Neurol Neurosurg Psychiatry 2003;74:1667–1673 Objective: To determine the role of CSF hypocretin-1 in narcolepsy with and without cataplexy, Kleine- Levin syndrome (KLS), idiopathic and other hypersomnias, and several neurological conditions. Patients: 26 narcoleptic patients with cataplexy, 9 narcoleptic patients without cataplexy, 2 patients with abnormal REM-sleep-associated hypersomnia, 7 patients with idiopathic hypersomnia, 2 patients with post-traumatic hypersomnia, 4 patients with KLS, and 88 patients with other neurological disorders. See end of article for Results: 23 patients with narcolepsy-cataplexy had low CSF hypocretin-1 levels, while one patient had a authors’ affiliations normal hypocretin level (HLA-DQB1*0602 negative) and the other two had intermediate levels (familial ....................... forms). One narcoleptic patient without cataplexy had a low hypocretin level. One patient affected with Correspondence to: post-traumatic hypersomnia had intermediate hypocretin levels. The KLS patients had normal hypocretin Dr Yves Dauvilliers, Service levels while asymptomatic, but one KLS patient (also affected with Prader-Willi syndrome) showed a de Neurologie B, Hoˆpital Gui-de-Chauliac, twofold decrease in hypocretin levels during a symptomatic episode. Among the patients without 80 avenue Augustin Fliche, hypersomnia, two patients with normal pressure hydrocephalus and one with unclear central vertigo had 34295 Montpellier cedex intermediate levels. -

Common Myths About Sleep
SHF-SleepMyths-0112 27/1/12 3:18 PM Page 1 Common Myths About Sleep 1 Myth: The brain shuts down 3 Myth: Sleeping in on the and is inactive during sleep weekend prevents the effects In fact, the brain is very busy during sleep. Among other of sleep loss during the next things, it sorts and processes information on what was going week on that day. Then it cements these into your long term memory. This is a vital for learning and memory. Sleeping in will work when you haven’t been sleeping enough and you need to pay back a sleep debt. But you can’t bank sleep in advance. If you are behind on sleep, it 2 Myth: You can train yourself to will affect how well your mind works. Things won’t get better until you catch up on the sleep that you missed. get by with less sleep Regular sleep habits help build a good, strong sleep-wake pattern and keep you at the top of your game. How much sleep is needed each night varies between people. The average adult needs about 8 hours a night. Some need less, others more. Most of us know from our own experience how much we need to feel good the next day. 4 Myth: Daytime sleepiness will Getting less than this builds a sleep debt. Sooner or later this always get better if you spend will have to be paid back. You might not notice missing an hour or so every now and then, but it is not good to make a more time in bed habit of it.