Botanical Supplements for Gut Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Habitat Management Plan Phoenix Park 2008
Phoenix Park Habitat Management Plan Report commissioned by The Office of Public Works Mary Tubridy and Associates August 2008 Clontarf, Dublin 3 Tel 01-8333195 Phoenix Park Habitat Management Plan Contents Acknowledgements 3 Summary 4 1 Methodology 1.1 Habitat map 5 1.2 Management plan 5 2 Habitats 2. 1 Introduction 6 2.2 Wetland habitats 8 2.2.1 Flora 8 2.2.2 Biodiversity evaluation 9 2. 3 Grasslands 9 2.3.1 Flora 10 2.3.2 Biodiversity evaluation 11 2.4 Woodlands 11 2.4.1 Flora 11 2.4.2 Biodiversity evaluation 12 3 Habitat Management Plan 3.1 Objectives 13 3.2 Short to medium term actions 13 3.2.1 Habitat and species diversity 13 3.2.2 Grassland management 14 3.2.3 Woodland management 14 3.2.4 Wetland management 15 3.2.5 Mammals and birds 15 3.2.6 Research, monitoring and education 16 References 17 Appendix 1 Notes on habitat diversity in Dublin 18 Mary Tubridy and Associates 2 Phoenix Park Habitat Management Plan Acknowledgements The study benefited from practical assistance, information and advice from the following organizations and individuals: OPW staff involved in the management of the Phoenix Park, particularly Dr John McCullen Margaret Gormley and Gabriel Gleeson. Managers of enclosures (Áras, United States Ambassador’s Residence, Zoo). Drs Linda Patton and Maurice Eakin of the National Parks and Wildlife Service The researchers who carried out specialist studies. They include Tom Hayden and his students from UCD (fallow deer, grey squirrel and mammals); Joe Caffrey and John Coyne of the Central Fisheries Board (freshwater habitats); Olivia Crowe and colleagues in BirdWatch Ireland (birds), Paul Scott of ScottCawley Ltd (bats) and Robbie Meehan (geodiversity). -

Filipendula Ulmaria (L.) Maxim
6 May 2020 EMA/HMPC/595722/2019 Committee on Herbal Medicinal Products (HMPC) Addendum to Assessment report on Filipendula ulmaria (L.) Maxim. (= Spiraea ulmaria L.), herba Rapporteur(s) B Kroes Assessor(s) Jan van der Nat Peer-reviewer J Wiesner HMPC decision on review of monograph Filipendula ulmaria (L.) Maxim. (= Spiraea 30 January 2018 ulmaria L.), herba adopted on July 2011 Call for scientific data (start and end date) From 30 April 2018 to 31 July 2018 Adoption by Committee on Herbal Medicinal 6 May 2020 Products (HMPC) Review of new data on Filipendula ulmaria (L.) Maxim., herba Periodic review (from 2011 to 2018) Scientific data (e.g. non-clinical and clinical safety data, clinical efficacy data) Pharmacovigilance data (e.g. data from EudraVigilance, VigiBase, national databases) Scientific/Medical/Toxicological databases: Scopus, PubMed, Embase, ToxNet Other Regulatory practice Old market overview in AR (i.e. products fulfilling 30/15 years on the market) New market overview (including pharmacovigilance actions taken in member states) – information from Member States (reporting between November 2018 and January 2019): Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Referral Ph.Eur. monograph: Filipendulae ulmariae herba 04/2013:1868 Currently: request for revision: replacement of hexane in TLC identification. Other Consistency (e.g. scientific decisions taken by HMPC) Public statements or other decisions taken by HMPC Consistency with other monographs within the therapeutic area Other Availability of new information (i.e. -

Meadowsweet 2015
ONLINE SERIES MONOGRAPHS The Scientific Foundation for Herbal Medicinal Products Filipendulae ulmariae herba Meadowsweet 2015 www.escop.com The Scientific Foundation for Herbal Medicinal Products FILIPENDULAE ULMARIAE HERBA Black Cohosh 2015 ESCOP Monographs were first published in loose-leaf form progressively from 1996 to 1999 as Fascicules 1-6, each of 10 monographs © ESCOP 1996, 1997, 1999 Second Edition, completely revised and expanded © ESCOP 2003 Second Edition, Supplement 2009 © ESCOP 2009 ONLINE SERIES ISBN 978-1-901964-37-0 Filipendulae ulmariae herba - Meadowsweet © ESCOP 2015 Published by the European Scientific Cooperative on Phytotherapy (ESCOP) Notaries House, Chapel Street, Exeter EX1 1EZ, United Kingdom www.escop.com All rights reserved Except for the purposes of private study, research, criticism or review no part of this text may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, without the written permission of the publisher. Important Note: Medical knowledge is ever-changing. As new research and clinical experience broaden our knowledge, changes in treatment may be required. In their efforts to provide information on the efficacy and safety of herbal drugs and herbal preparations, presented as a substantial overview together with summaries of relevant data, the authors of the material herein have consulted comprehensive sources believed to be reliable. However, in view of the possibility of human error by the authors or publisher of the work herein, or changes in medical knowledge, neither the authors nor the publisher, nor any other party involved in the preparation of this work, warrants that the information contained herein is in every respect accurate or complete, and they are not responsible for any errors or omissions or for results obtained by the use of such information. -

Top 6 Herbs to Heal Pain and Inflammation in Horses
Top 6 Herbs to Heal Pain and Inflammation in Horses Discover the Top Herbs to Manage your Horse’s Pain and Inflammation - Naturally Why Herbal Medicine? It is understood that animals have been self-medicating with herbs throughout time. In fact, the same can be said for humans. Did you know that many pharmaceutical drugs have been developed as a result of studying traditional herbal medicines, extracting what are considered to be the key chemical constituents, synthetically manufacturing those chemicals and prescribing them at high doses? In many developing countries, a large portion of the population still rely heavily on traditional practitioners and medicinal plants to meet their primary health care needs, even where more modern Western medical systems are available. So why use herbal medicines when there are pharmaceutical alternatives? What we are unable to replicate in pharmaceutical drugs is the manner in which the multitude of different phytochemicals found in plant medicines work together synergistically to heal the body. Taking a single chemical component from a plant and prescribing it at a high dose often produces side effects that are not evident when the medicine is taken in plant form, as plants tend to contain just the right balance of a range of phytochemicals. This is not to say that plant medicines have no side effects, as some are toxic when prescribed incorrectly. Another factor to consider is cost. Herbal medicines are often significantly cheaper than pharmaceutical medicines, largely because herbal medicines cannot be patented. In some cases, you can even grow fresh herbs in your own home. -

Anti-Urolithiatic Activity of Filipendula Ulmaria Leaf Extracts in Male Albino Rats
CATRINA (2020), 22(1): 49-55 © 2020 BY THE EGYPTIAN SOCIETY FOR ENVIRONMENTAL SCIENCES Anti-Urolithiatic Activity of Filipendula ulmaria Leaf Extracts in Male Albino Rats Rabab M. Aljarari*1 and Muna O. Alamoudi2 1 Biology Department, Faculty of Science, Jeddah University, Jeddah - Saudi Arabia 2 Biology Departments, Faculty of Science, Hail University, Hail City - Saudi Arabia ABSTRACT Filipendula ulmaria L. is a perennial herb that can be found in regions with higher humidity in Asia, and Europe. The herb is used medicinally as drugs for several purposes such as facilitating renal elimination functions and many other biological activities. Therefore, the present study was focus on the anti-Urolithiatic activity of Filipendula ulmaria leaf extracts using Albino rats as a model. The influence of oral administration of aqueous, methanolic and ethanolic extracts of Filipendula ulmaria leaves on accumulated calcium oxalate urolithiasis has been investigated. Nephrolithiasis was induced in the rats by oral administration of ethylene glycol (0.75%) in drinking water for 28 days. Animals were divided into nine groups, each containing six rats. Group 1 received purified water as negative control; group 2 received ethylene glycol in drinking water; group 3 received cystone as curative agent, groups 4-9 received Filipendula ulmaria extracts in dosages of 100 and 200 mg/kg body weight, respectively. The aqueous and methanolic Leaf-extracts showed significant reduction in the urine parameters compared to ethanolic extracts. However, using the cystone showed highest reduction rate compared to the leaf-extracts using different solvents. Cystone also recorded higher impact on the serum parameters compared to the extract used. -

Site Synopsis
SITE SYNOPSIS SITE NAME : LOWER RIVER SUIR SITE CODE : 002137 This site consists of the freshwater stretches of the River Suir immediately south of Thurles, the tidal stretches as far as the confluence with the Barrow/Nore immediately east of Cheekpoint in Co. Waterford and many tributaries including the Clodiagh in Co. Waterford, the Lingaun, Anner, Nier, Tar, Aherlow, Multeen and Clodiagh in Co. Tipperary. The Suir and its tributaries flows through the counties of Tipperary, Kilkenny and Waterford. Upstream of Waterford city, the swinging meanders of the Suir criss- cross the Devonian sandstone rim of hard rocks no less than three times as they leave the limestone-floored downfold below Carrick In the vicinity of Carrick-on-Suir the river follows the limestone floor of the Carrick Syncline. Upstream of Clonmel the river and its tributaries traverse Upper Palaeozoic Rocks, mainly the Lower Carboniferous Visean and Tournaisian. The freshwater stretches of the Clodiagh River in Co. Waterford traverse Silurian rocks, through narrow bands of Old Red Sandstone and Lower Avonian Shales before reaching the carboniferous limestone close to its confluence with the Suir. The Aherlow River flows through a Carboniferous limestone valley, with outcrops of Old Red Sandstone forming the Galtee Mountains to the south and the Slievenamuck range to the north. Glacial deposits of sands and gravels are common along the valley bottom, flanking the present-day river course. The site is a candidate SAC selected for the presence of the priority habitats on Annex I of the E.U. Habitats Directive - alluvial wet woodlands and Yew Wood. -

Dorset Moths (Vc9) Annual Report 2019
DORSET MOTHS (VC9) ANNUAL REPORT 2019 Paul Butter, Phil Sterling, Mike Hetherington, Jack Oughton & Alison Stewart 1 CONTENTS Introduction Mike Hetherington 2 Highlights of the Year Jack Oughton 4 Summary of 2019 Records Alison Stewart 6 List of Recorders 8 Macro Moths 2019 Paul Butter & Mike Hetherington 9 Micro Moths 2019 Phil Sterling 27 Migrant Moth Report 2019 Paul Butter & Jack Oughton 36 Dearth of Daytime Observations Paul Butter 39 Dorset Moths Annual Meeting 2019 Mike Hetherington 40 Grass Webworms in Dorset 2019 Mike Hetherington 41 The Geometrician Grammodes stolida – a first for Dorset, recorded day-flying on Portland on 24/09. Photo of that record © Bob Johnson. Moitrelia obductella – another Dorset first for the year, found as larvae on Marjoram Origanum vulgare Wyke Regis on 20/06. Photo of adult raised from larva by Dave Foot © Paul Harris. Front cover images © Mike Hetherington (Cream-spot Tiger & Elephant Hawk-moth), Paul Butter (Forester), Paul Harris (Ancylolomia tentaculella). DMG Logo © Chris Manley 2 INTRODUCTION Welcome to the Dorset Moths Annual Report for 2019. As many of you will be aware, a new verification team took over when Les Evans-Hill stepped down as County Moth Recorder at the end of 2016. The current team members are: Adrian Bicker (Living Record), Terry Box, Paul Butter, Pete Forrest, Julian Francis, Mike Hetherington, Tom Morris, Jack Oughton, Phil Sterling (micro moth County Moth Recorder) and Alison Stewart (Dorset Environmental Records Centre). After addressing a backlog in the verification of records for 2017 and 2018 the team is now in a position to produce an Annual Report for 2019. -
Assessment Report on Filipendula Ulmaria (L.) Maxim., Herba and Filipendula Ulmaria (L.) Maxim., Flos
12 July 2011 EMA/HMPC/434892/2010 Committee on Herbal Medicinal Products (HMPC) Assessment report on Filipendula ulmaria (L.) Maxim., herba and Filipendula ulmaria (L.) Maxim., flos Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use) Final Herbal substance(s) (binomial scientific name of Filipendula ulmaria (L.) Maxim., herba the plant, including plant part) Filipendula ulmaria (L.) Maxim., flos Herbal preparation(s) Herb: a) Comminuted herbal substance b) Powdered herbal substance c) Tincture (ratio of herbal substance to extraction solvent 1:5), extraction solvent ethanol 45% (V/V) Flowers: Comminuted herbal substance Pharmaceutical forms Herb: Comminuted herbal substance as herbal tea for oral use. Powdered herbal substance in solid dosage forms for oral use. Herbal preparation in liquid dosage form for oral use. Flowers: Comminuted herbal substance as herbal tea for oral use. Rapporteur Dr Emiel van Galen Assessors Dr Emiel van Galen Dr Burt Kroes Dr Jan van der Nat 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7523 7051 E-mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2011. Reproduction is authorised provided the source is acknowledged. Table of contents 1. Introduction.......................................................................................................................3 1.1. Description of the herbal substance(s), herbal preparation(s) or combinations thereof .3 1.2. Information about products on the market in the Member States .............................. 4 1.3. Search and assessment methodology.................................................................... 7 2. Historical data on medicinal use ........................................................................................7 2.1. -

(Filipendula Ulmaria (L.) Maxim.) Is Shaped by Phytosociological Habitats
molecules Article Insight into the Way the Content of Biologically Active Compounds in Meadowsweet Inflorescences (Filipendula ulmaria (L.) Maxim.) Is Shaped by Phytosociological Habitats Kinga Stawarczyk 1, Aleksandra Chrupek 2, Agnieszka S˛ekara 2 , Michał Gostkowski 3 and Małgorzata Karbarz 1,* 1 Department of Biology, Institute of Biology and Biotechnology, University of Rzeszow, Pigonia Street 1, 35-310 Rzeszów, Poland; [email protected] 2 Department of Horticulture, Faculty of Biotechnology and Horticulture, University of Agriculture in Krakow, 29 Listopada 54, 31-425 Krakow, Poland; [email protected] (A.C.); [email protected] (A.S.) 3 Department of Econometrics and Statistics, Institute of Economics and Finance, Warsaw University of Life Sciences-SGGW, 02-776 Warsaw, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-178518754 Abstract: A collection of herbs from the natural environment remains not only a source of raw mate- rial but also provides evidence of chemical differentiation of the local populations. This work aimed at performing a phytosociological analysis of seven different stands of meadowsweet (Filipendula ul- maria (L.) Maxim.) occurrence. A determination of total phenolic compounds and salicylates and the antioxidant activity of dried meadowsweet inflorescences (Flos ulmariae) was also performed. Active Citation: Stawarczyk, K.; Chrupek, chemical compounds in F. ulmaria inflorescences were related to chemotype and diversified between A.; S˛ekara,A.; Gostkowski, M.; investigated populations. Geographical distance and variation in phytosociological locations affected Karbarz, M. Insight into the Way the chemical composition in different ways, shaping the content of biochemical compounds crucial for Content of Biologically Active herbal material quality. -

Madryn's Millefleurs – Meadowsweet
MADRYN'S MILLEFLEURS MEADOWSWEET Filipendula ulmaria "Let us seek, thou and I , by the magic of enchantment to conjure a wife...out of flowers...And then they took the flowers of the oak, and the flowers of the broom and the flowers of the meadowsweet, and from those they called forth the very fairest and best endowed maiden that mortal ever saw...and named her Blodeuedd..." Translated from The Mabinogion 1300-1325 I first encountered meadowsweet as a mesmerised 11 year old, reading the wonderful children's tale "The Owl Service" by Alan Garner. In this mystical story, three contemporary teenagers holidaying in the Welsh mountains are bound into an ancient legend of the Mabingion, the 11th century Celtic story of Blodeuedd, a woman conjured out of flowers, and given in marriage to a man she did not love. When she fell in love with another man, for whom she was not intended, she betrayed her husband. Husband and lover were then set against each other, resulting in both their deaths. As punishment, the wizard who had made Blodeuedd turned her into an owl, "And because of the dishonour thou hast done to {thy husband} thou art never to dare show thy face in the light of day...there {shall} be emnity between thee and all birds...thou {shall} be forever called Blodeuedd" Translated from the Mabinogion. In parts of Wales the owl is still known as Blodueuedd to this day. Throughout the Owl Service novel which held me so transfixed all those years ago, meadowsweet is a predominent feature, both by colouring the landscape in which the story unfolds, and in the conjuring of the beautiful Blodeuedd for her lord. -

Comparison of the Anti-Inflammatory and Immunomodulatory Mechanisms of Two Medicinal Herbs: Meadowsweet (Filipendula
International Journal of Plant, Animal and Environmental Sciences DOI: 10.26502/ijpaes.002 Research Article Comparison of The Anti-Inflammatory and Immunomodulatory Mechanisms of Two Medicinal Herbs: Meadowsweet (Filipendula ulmaria) and Harpagophytum (Harpagophytum procumbens) Juliette Cholet1*, Caroline Decombat1†, Marjolaine Vareille-Delarbre1,† , Maël Gainche2, Alexandre Berry1, Clémence Ogéron2, Isabelle Ripoche2, Laetitia Delort1, Marion Vermerie1, Didier Fraisse1, Catherine Felgines1, Edwige Ranouille3, Jean-Yves Berthon3, Albert Tourette4, Yves Troin2, François Senejoux1, Pierre Chalard2, Florence Caldefie-Chezet1 1Université Clermont Auvergne, INRA, UNH, Unité de Nutrition Humaine, CRNH Auvergne, F-63000, Clermont-Ferrand, France 2Université Clermont Auvergne, CNRS, SIGMA Clermont, ICCF, F-63000 Clermont-Ferrand, France 3Greentech, Biopôle Clermont-Limagne, 63360 Saint-Beauzire, France 4AltoPhyto, 7 rue des gargailles, 63370 Lempdes, France †Equal contributions *Corresponding Author: Juliette Cholet, Université Clermont Auvergne, INRA, UNH, Unité de Nutrition Humaine, CRNH Auvergne, F-63000, Clermont-Ferrand, France, Tel: +33-4-73-17-79-72; E-mail: [email protected] Received: 24 June 2019; Accepted: 10 July 2019; Published: 22 July 2019 Abstract Background: Meadowsweet (Filipendula ulmaria) and Harpagophytum (H. procumbens) are two medicinal herbs traditionally used for their anti-inflammatory effect. Nonetheless, if the effects of the single compounds isolated from these plants have been well described, little is known about the molecular mechanisms behind whole extracts. Methods: We studied and compared the effects of methanolic extracts from the aerial parts of F. ulmaria (FUE) and from the roots of H. procumbens (HPE) on different markers of inflammation such as antioxidant capacity, leukocyte ROS production, COX-2/PGE2 pathway or cytokine secretions. Results: FUE proved to be better than HPE in terms of antioxidant capabilities. -
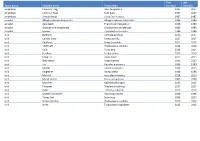
Taxon Group Common Name Taxon Name First Recorded Last
First Last Taxon group Common name Taxon name recorded recorded amphibian Common Frog Rana temporaria 1987 2017 amphibian Common Toad Bufo bufo 1987 2017 amphibian Smooth Newt Lissotriton vulgaris 1987 1987 annelid Alboglossiphonia heteroclita Alboglossiphonia heteroclita 1986 1986 annelid duck leech Theromyzon tessulatum 1986 1986 annelid Glossiphonia complanata Glossiphonia complanata 1986 1986 annelid leeches Erpobdella octoculata 1986 1986 bird Bullfinch Pyrrhula pyrrhula 2016 2017 bird Carrion Crow Corvus corone 2017 2017 bird Chaffinch Fringilla coelebs 2015 2017 bird Chiffchaff Phylloscopus collybita 2014 2016 bird Coot Fulica atra 2014 2014 bird Fieldfare Turdus pilaris 2015 2015 bird Great Tit Parus major 2015 2015 bird Grey Heron Ardea cinerea 2013 2017 bird Jay Garrulus glandarius 1999 1999 bird Kestrel Falco tinnunculus 1999 2015 bird Kingfisher Alcedo atthis 1986 1986 bird Mallard Anas platyrhynchos 2014 2015 bird Marsh Harrier Circus aeruginosus 2000 2000 bird Moorhen Gallinula chloropus 2015 2015 bird Pheasant Phasianus colchicus 2017 2017 bird Robin Erithacus rubecula 2017 2017 bird Spotted Flycatcher Muscicapa striata 1986 1986 bird Tawny Owl Strix aluco 2006 2015 bird Willow Warbler Phylloscopus trochilus 2015 2015 bird Wren Troglodytes troglodytes 2015 2015 bird Yellowhammer Emberiza citrinella 2000 2000 conifer Douglas Fir Pseudotsuga menziesii 2004 2004 conifer European Larch Larix decidua 2004 2004 conifer Lawson's Cypress Chamaecyparis lawsoniana 2004 2004 conifer Scots Pine Pinus sylvestris 1986 2004 crustacean