Assessment of Water Quality Parameters of the River Brahmani at Rourkela
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

INTEGRATED TRIBAL DEVELOPMENT AGENCY, PANPOSH Tel.No
INTEGRATED TRIBAL DEVELOPMENT AGENCY, PANPOSH Tel.No. - 0661-2501011, e-mail- [email protected] No. __2278/ Date: _15.12.2016___/ e-Procurement Notice Bid Identification No. ITDAPAN-02/2016-2017 1. The Project Administrator, ITDA, Panposh invites Percentage Rate bids in online mode for the works as detailed in the table, from the class of eligible contractors as mentioned in column-5 (five) registered with the State Governments and Contractors of equivalent Grade / Class Registered with Central Government / MES / Railways or in equivalent rank may participate in the tender but successful bidder has to register under the State PWD before signing of the agreement. The proof of registration from the appropriate authority shall be enclosed along with the bid. 2. The bidders may submit bids for the following works. Sl Name of the Work Approx. Period Class of Bid Cost of Last date & time of No Amount put of Contractor Security/ bid online receipt of to tender Completion EMD document bid (in Rs.) (in Rs.) 1 2 3 4 5 6 7 8 1 Constn. of Addl. Class room 2539485/- Four C&B 25395/- 6300/- 26.12.16 at Kalosihira S/S under months up to 5.00 PM Kuarmunda block 2 Constn. of Addl. Class room 1620698/- Four D&C 16207/- 6300/- 26.12.16 at KokeramaS/S under months up to 5.00 PM Nuagaon block 3 Constn. of Addl. Class room 1523164/- Four D&C 15232/- 6300/- 26.12.16 at Lanjiberna S/S under months up to 5.00 PM Kuarmunda block 4 Constn. of Addl. -

List of the State Govt.Employees to Retire on 31.05.2021
LIST OF THE STATE GOVT.EMPLOYEES TO RETIRE ON 31.05.2021 SERIES ACCOUNT_NO SUBSCR_NAME DOB DOR TRY DDO AJO 5152 NABARAJ PRADHAN 16-May-61 31/05/2021 PLB Additional Muncif Judicial Magistrate,G Udyagiri AJO 5364 BARIK PRASANTA KUMAR 24-May-61 31/05/2021 NRG Registrar Civil Courts,Nabarangpur AJO 5492 BARIK GANGADHAR 17-May-61 31/05/2021 PRI Registrar Civil Courts,Puri AJO 5545 NAYAK SANYASI 15-May-61 31/05/2021 GJP Registrar Civil Courts Gajapati,Paralakhemundi AJO 5573 SAHU SACHIDANANDA 22-May-61 31/05/2021 SNP Registrar Civil Courts,Sonepur AJO 5685 TARAI KAILASH CHANDRA 24-May-61 31/05/2021 GJP Registrar Civil Courts Gajapati,Paralakhemundi AJO 5791 DAS SAROJ KUMAR 02-May-61 31/05/2021 CTC Registrar Civil Court,Cuttack AJO 5873 PATTANAYAK KHIROD RANJAN 11-May-61 31/05/2021 BDK Registrar Civil Courts,Bhadrak AJO 5904 SARANGI PATITAPABAN 07-May-61 31/05/2021 CTC Establishment Officer Odisha High Court,Cuttack AJO 5927 TRIPATHY JAYANTA KUMAR 13-May-61 31/05/2021 DKL Judge Family Court,Dhenkanal AJO 5959 BISWAS MAHITOSH 02-May-61 31/05/2021 PRI Registrar Civil Courts,Puri AJO 6078 MISHAR LAXMIDHAR 28-May-61 31/05/2021 PRI Registrar Civil Courts,Puri AJO 6125 ROUTA SIMANCHALA 01-Jun-61 31/05/2021 DKL Civil Judge (Jr. Divn.) cum J.M.F.C.,Bhuban AJO 6182 PANDA BHAGABAN 26-May-61 31/05/2021 PRI Judge Family Court Puri,Puri AJO 6443 BEHERA SURESH CHANDRA 02-May-61 31/05/2021 MKG Registrar Civil Courts,Malkangiri AJO 6581 MISHRA RABI NARAYAN 03-May-61 31/05/2021 KPT Civil Judge Senior Division,Koraput AJO 6805 MOHANTY AMRENDRA KUMAR 04-May-61 -

NEW RAILWAYS NEW ODISHA a Progressive Journey Since 2014 Sundargarh Parliamentary Constituency
TIVE Y INDICA MAP IS ONL Shri Narendra Modi Hon'ble Prime Minister NEW RAILWAYS NEW ODISHA A progressive journey since 2014 Sundargarh Parliamentary Constituency SUNDARGARH RAILWAYS’ DEVELOPMENT IN ODISHA (2014-PRESENT) SUNDARGARH PARLIAMENTARY CONSTITUENCY A. ASSEMBLY SEGMENTS : Talsara, Sundargarh, Biramitrapur, Raghunathpali, Rourkela, Rajgangpur, Bonai RAILWAY STATIONS COVERED : Rourkela, Rajgangpur, Bamra, Bondamunda, Garposh, Kanshbahal, Panposh, Kalunga, Tangarmunda, Sonakhan, Sagra, Daghora, Bisra, Nuagaon, Jamga, Himgir, Chandiposi, Kuarmunda, Bimlagarh Junction, Birmitrapur, Barsuan, Dumerta, Lathikata, Dharuadihi, Dhutra, Karampada, Barajamda, Gua, Goilkera, Posoita, Manoharpur, Jaraikela, Bhalulata, Orga B. WORKS COMPLETED IN LAST FIVE YEARS : B.1. New Trains and Stoppages / Extension / Increase in Frequency : Train No. 58660-58659, Rourkela- Hatia-Rourkela passenger started from 09.02.2015. Train No. 78103-78104 Rourkela- Sambalpur-Rourkela DMU started from 07.06.2015. Train No. 12101-12102, Jnaneswari Deluxe Howrah-Lokmanya Tilak Terminus-Howrah provided stoppage at Jharsuguda from 09.05.2017. Train No. 18110-18109, Jammu Tawi MURI Rourkela Express extended from Jammu Tawi to Rourkela and further extended upto Sambalpur w.e.f.12.08.2017. Train No. 18417-18418, Rajya Rani Exp from Rourkela to Bhubaneswar extended upto Gunupur from 21.03.2017. Train No. 18415/18416 Puri-Barbil-Puri Express has been extended upto Rourkela. Train No. 18451/18452 Tapaswini Express has been provided additional stoppage at Kalunga. Train No. 18107/18108 Rourkela-Jagdalpur-Rourkela Express has been provided additional stoppage at Rajgangpur. Train No. 18108/18107 Rourkela - Koraput - Rourkela Express extended upto Jagdalpur. Frequency of 18117/18118 Rourkela-Gunupur-Rourkela Rajyarani Express has been increased to run Daily. B.2. Improvement of Passenger Amenities : Escalators at important stations - 2 Nos at Rourkela at a cost of `1.060 Crore. -

State Institute of Hotel Management Balangir Odisha
STATE INSTITUTE OF HOTEL MANAGEMENT TITILAGARH ROAD, BALANGIR – 767001 (ODISHA) Trade : Homestay (Multi Skilled Caretaker) Duration of the Training : 04.09.2018 To 11.10.2018 SL. NAME OF THE FATHER’S NAME ADDRESS MOBILE NO. NO. CANDIDATE AT-JALDA ‘A’ BLOCK, P.O-JALDA Mr SARASWATI 01 Ms ARATI SINGH ‘C’ BLOCK, DIST-SUNDERGARH, PIN- SINGH 769043 (ODISHA) AT/P.O-TANGARPALI, DIST- Ms BANGI Mr DHANANJAY 02 SUNDERGARH, PIN-769007 MURMU MURMU (ODISHA) AT-ABC COLONY, P.O-UPPER Mr SALKHAN BALIJODI, HATIBANDHA, DIST- 03 BIRDA SOREN 6370968196 SOREN SUNDERGARH, PIN-769016 (ODISHA) AT-MANKO (TANKATOLA), P.O- Ms CHARIA 04 Mr DHADU EKKA MANKO, DIST-SUNDERGARH, PIN- EKKA 770037 (ODISHA) AT-KATIN (TOLA), HATIBANDHA, Ms DILESWARI Mr CHARKHA P.O-ASURCHHAPAL, DIST- 05 ORAM ORAM SUNDERGARH, PIN-769043 (ODISHA) AT-JALDA ‘B’ BLOCK, P.O-JALDA Ms DIVYA RANI Mr NARAYAN 06 ‘C’ BLOCK, DIST-SUNDERGARH, PIN- KISHAN KISHAN 769043 (ODISHA) AT-BURUDI BASTI, P.O-TANGARPALI, Ms DULARI Mr DHANJAYA 07 DIST-SUNDERGARH, PIN-769007 MURMU MURMU (ODISHA) Mr PRATAP AT-B/150, SEC-22, P.O-TANGARPALI, HRUSHIKESH 08 CHANDRA DIST-SUNDERGARH, PIN-769007 8093944309 SAHOO SAHOO (ODISHA) AT-JALDA ‘B’ BLOCK, P.O-JALDA Mr SUKLA 09 Ms JULI KISSAN ‘C’ BLOCK, DIST-SUNDERGARH, PIN- KISSAN 769043 (ODISHA) AT-MANKO (TONKATOLI), P.O- 10 Ms KOILI EKKA Mr DALE EKKA MANKO, DIST-SUNDERGARH, PIN- 770037 (ODISHA) AT-GHD, JALDA ‘C’ BLOCK, P.O- Mr GIRIJA Ms KRISHNA JALDA ‘C’ BLOCK, DIST- 11 PRASAN PATTNAYAK SUNDERGARH, PIN-769043 PATTNAYAK (ODISHA) AT-DEOGAON, P.O-PANPOSH, DIST- Ms LAXMI 12 Mr RAM BARMA SUNDERGARH, PIN-769004 BARMA (ODISHA) SL. -

26-06-2021 Rgh, Rourkela
DEPARTMENT OF MICROBIOLOGY ISPAT GENERAL HOSPITAL SAIL, RSP, ROURKELA COVID-19 (RT-PCR) Test Report Date and time of reporting 26.06.2021; 07:00 P.M. Address of the referring facility/Hospital Rourkela Govt. Hospital, Rourkela SPECIMEN DETAILS Date & Time of sample collection 25.06.2021; 09:00 A.M. Date & Time of receipt of specimen 26.06.2021; 09:30 A.M. Condition of specimens received / Quality on arrival Acceptable REPORTING DETAILS Result Sr. Date of sample Sample ID Patient Name Age Sex Specimen type Mobile No. Address No. testing 2019-nCoV Kahachua,Jareikela, Rajgangpur 1 Cov/80992 Pramila Toppo 26 F Nasal Swab 25.06.2021 Negative NA Pin:770017 2 Cov/80993 John Patra 40 M Nasal Swab 25.06.2021 Negative NA Barupada, Rajgangpur Pin:770017 Swadhin Colony, Rajgangpur 3 Cov/80994 Satyajeet Pradhan 20 M Nasal Swab 25.06.2021 Negative NA Pin:770017 4 Cov/80995 Premsila Xess 25 F Nasal Swab 25.06.2021 Negative NA Liploi, Rajgangpur Pin:770017 Spinning Mill Colony, Rajgangpur 5 Cov/80996 Saroj Kumar Jena 42 M Nasal Swab 25.06.2021 Negative NA Pin:770017 Ocl Colony Market, Rajgangpur 6 Cov/80997 Tanushree Chakrabarty 34 F Nasal Swab 25.06.2021 Negative NA Pin:770017 7 Cov/80998 Bibek Swah 22 M Nasal Swab 25.06.2021 Negative NA Ward No-16 Pin:770017 8 Cov/80999 Merry Bada 27 F Nasal Swab 25.06.2021 Negative NA Kumarkela, Rajgangpur Pin:770017 9 Cov/81073 Sashi Bhusan Ekka 34 M Nasal Swab 26.06.2021 Negative 7898740075 Laing CHC Rajgangpur 10 Cov/81074 Nabin Ranjan Sahoo 46 M Nasal Swab 26.06.2021 Negative 9776906890 Laing CHC Rajgangpur 11 -

Sl. No. TIN Name of the Dealer Address 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 LIST of DEALERS SELECTE
LIST OF DEALERS SELECTED FROM SUNDERGARH RANGE FOR TAX AUDIT DURING 2011-12 Sl. TIN Name of the dealer Address No. 1 21302000022 L & T LTD. KANSBAHAL, SUNDARGARH 2 21072000003 EAST INDIA STEEL PVT.LTD. INDUSTRIAL AREA,ROURKELA 3 SHREE GANESH METALICK 21022000079 LTD. HARIHAR PUR,KUARMUNDA 4 21352000043 PRABHU SPONGE IRON(P)LTD OCL APPROACH ROAD,ROURKELA 5 KOSHALA PLOT NO- 10/310,, PANPOSH 21812003961 AUTOMOBILES(P)LTD. ROAD,ROURKELA 6 21842000016 GRUHASTI UDYOG CHHEND BASTI, ROURKELA 7 PLOT NO-98,GOIBHANGA, INDUSTRIAL 21382000075 Time Steel & Power Ltd. ESTATE,, SUNDARGARH 8 BAJRANGA STEEL & ALLOYES 21082000046 PVT.LTD. PLOT NO-31, GOBHANGA, SUNDARGARH 9 UTKAL FLOUR MILLS(RKL) 21742000071 PVT.LTD. INDUSTRIAL AREA,, SUNDARGARH 10 21452003092 T.R.CHEMICALS LTD. BARPALLI,RAJGANGPUR 11 BARPALI KESARMAL(RAJGANGPUR) 21632000083 SURAJ PRODUCTS LTD. ROURKELA SUNDARGRAH 12 P-25,CTS, P-25,CTS, SUNDARGARH, 21312000065 M/S.SHIVA CEMENT LTD ROURKELA 13 RAJGANGPUR, RAJGANGPUR, 21062001318 HARI MACHINES L.T.D RAJGANGPUR, SUNDARGARH, 770017 14 IDCO PLOT NO- 76, INDUSTRIAL ESTATE, 21182000088 UTKAL METALLICS LTD KALUNGA, SUNDARGARH, 15 RAMABAHAL, RAJGANGPUR, 21192000034 M/S SCAN STEELS LTD. RAJGANGPUR, SUNDARGARH, 770017 16 BIJABAHAL KUARMUNDA KUARMUNDA 21202000077 PAWANJOY SPONGE IRON LTD SUNDARGARH 17 M/S SARVESH REFRACTORIES KUARMUNDA, KUARMUNDA, 21372000032 LTD KUARMUNDA, SUNDARGARH 770039 18 BALLANDA BALLANDA BALANDA,RKL 21422000053 M/S.MAA TARINI (P) LTD SUNDARGARH 19 SHREE RAM SPONGE BILAIGARH, BILAIGARH, BILAIGARH, 21442003243 &STEEL(P) LTD SUNDARGARH, 770034 20 BARPALI, BARPALLI, RAJGANGPUR, 21452003092 T.R. CHEMICALS LIMITED SUNDARGARH, 770017 21 RAJGANGPUR RAJGANGPUR 21482002542 M/S SHREE CHEM RESIN LTD . RAJGANGPUR SUNDARGARH 22 GOIBHARGA,KALUNGA 21592000008 KALINGA SPONGEIRONLTD GOIBHARGA,KALUNGA ROURKELA 23 INDERA MOTORS (PROP. -

DEPARTMENT of MICROBIOLOGY COVID-19 (RT-PCR) Test Report
DEPARTMENT OF MICROBIOLOGY ISPAT GENERAL HOSPITAL SAIL, RSP, ROURKELA COVID-19 (RT-PCR) Test Report Date and time of reporting 02.08.2021; 06:30 P.M. Address of the referring facility/Hospital Rourkela Govt. Hospital, Rourkela SPECIMEN DETAILS Date & Time of sample collection 01.08.2021; 09:00 A.M. Date & Time of receipt of specimen 02.08.2021; 09:00 A.M. Condition of specimens received / Quality on arrival Acceptable REPORTING DETAILS Result Sr. Date of sample Sample ID Patient Name Age Sex Specimen type Mobile No. Address No. testing 2019-nCoV 1 Cov/113313 Jambu Behera 75 F Nasal Swab 02.08.2021 Negative 7682044045 KN/28 Chhend Rourkela 2 Cov/113314 Sanjib Maharana 28 M Nasal Swab 02.08.2021 Negative 7381236405 Fertilizer Rourkela 3 Cov/113315 Satyabrata Barik 62 M Nasal Swab 02.08.2021 Negative 9439833339 MIG-2/253 Chhend Rourkela 4 Cov/113316 Soumya Ranjan Barik 26 M Nasal Swab 02.08.2021 Negative 9437192142 Chhend Rourkela 5 Cov/113317 Netaji Jena 43 M Nasal Swab 02.08.2021 Negative 7377337718 RN Pali Rourkela 6 Cov/113318 Manoj Ku Naik 28 M Nasal Swab 02.08.2021 Negative 9353947590 MCR-193 Chhend Rourkela 7 Cov/113319 Seema Pradhan 22 F Nasal Swab 02.08.2021 Negative 7763867172 Chakradharpur Rourkela 8 Cov/113320 Paresh Ku Swain 22 M Nasal Swab 02.08.2021 Negative 9658605656 Fertilizer Rourkela 9 Cov/113321 Sanjeet Lakra 35 M Nasal Swab 02.08.2021 Negative 8342962319 Auto Colony Rourkela 10 Cov/113322 Thomas Lugun 25 M Nasal Swab 02.08.2021 Negative 7328006242 Jalda Rourkela 11 Cov/113323 Rajkishor Mohanta 36 M Nasal Swab 02.08.2021 -

Sundargarh District at a Glance
Govt. of India MINISTRY OF WATER RESOURCES CENTRAL GROUND WATER BOARD OF SUNDERGARH DISTRICT CENTRAL GROUND WATER BOARD SOUTH EASTERN REGION BHUBANESWAR March, 2013. 1 SUNDARGARH DISTRICT AT A GLANCE I. General Particulars (a) Location : 21o 35' and 22o 32' North latitudes 83o 32' and 85o 22' East longitudes (b) Area : 9,712 Km2 (c) District Head quarters : Sundargarh (d) Subdivision : 3(three) – 1. Sundargarh 2.Panposh 3.Bonaigarh (e)Tehsils 9(nine) (f) Blocks : 17(seventeen) 9.kutra 1.Balisankara 10.Lahunipada 2.Baragaon 11.Lathikata 3.Bisra 12.lephripada 4.Bonaigarh 13.Nuagaon 5.Gurundia 14.Rajgangapur 6.Hemagiri 15. Subdega 7.Koida 16. Sundargarh 8. Kuarmunda 17. Tangarpalli (g) Population : 20,80,664 (as per 2011 census) II Climatology : (a) Normal annual rainfall : 1647.6 mm (b) Maximum temperature : 48 o C (c) Minimum temperature : 6o C III Land use : (a) Total forest area : 146061 Ha (b) Net area sown : 257927Ha IV Irrigation potential created (source Kharif Rabi wise) (upto 2000-01) (a) Minor irrigation Projects : 15425 Ha 1358 Ha (flow) 2 (b) Lift Irrigation Projects : 6925 Ha 2884 Ha (c) Ground water Structures : 10277 Ha V Exploratory wells : Bore wells drilled by CGWB : 101 EW &26 OW under Normal Exploration Programme (As 0n 31.03.2011) VI Ground Water Resources a) Annual ground water : 1668914 ham resource assessed b) Annual ground water draft (for : 436202 ham all uses) c) Balance ground water : 1201460 ham resource for irrigation use VII Stage of ground water : 26.14% development 3 1.0. INTRODUCTION Sundargarh district with an area of 9712 sq km and population of 1830673 (as per 2001 census) is the northern most district of Orissa. -

Sundargarh.Pdf
TIVE Y INDICA MAP IS ONL Shri Narendra Modi Hon'ble Prime Minister NEW RAILWAYS NEW ODISHA A progressive journey since 2014 Sundargarh Parliamentary Constituency SUNDARGARH RAILWAYS’ DEVELOPMENT IN ODISHA (2014-PRESENT) SUNDARGARH PARLIAMENTARY CONSTITUENCY A. ASSEMBLY SEGMENTS : Talsara, Sundargarh, Biramitrapur, Raghunathpali, Rourkela, Rajgangpur, Bonai RAILWAY STATIONS COVERED : Rourkela, Rajgangpur, Bamra, Bondamunda, Garposh, Kanshbahal, Panposh, Kalunga, Tangarmunda, Sonakhan, Sagra, Daghora, Bisra, Nuagaon, Jamga, Hemagiri, Chandiposi, Kuarmunda, Bimlagarh Junction, Birmitrapur, Barsuan, Dumerta, Lathikata, Dharuadihi, Dhutra, Karampada, Barajamda, Gua, Goilkera, Posoita, Manoharpur, Jaraikela, Bhalulata, Orga B. WORKS COMPLETED IN LAST FOUR YEARS : B.1. New Trains and Stoppages / Extension : Train No. 58660-58659, Rourkela- Hatia-Rourkela passenger started from 09.02.2015. Train No. 78103-78104 Rourkela- Sambalpur-Rourkela DMU started from 07.06.2015. Train No. 12101-12102, JNANESWARI DELX Howrah- Lokmanya Tilak Term- Howrah provided stoppage at Jharsuguda from 09.05.2017. Train No. 18110-18109, JAT MURI ROU EXP extended from Jammu Tawi to Rourkela extended upto Sambalpur w.ef.12.08.2017. Train No. 18417-18418, Rajya Rani Exp from Rourkela to Bhubaneswar extended upto Gunupur from 21.03.2017. B.2. Improvement of Passenger Amenities : Escalators at important stations - 2 Nos at Rourkela at a cost of `1.060 Crore. Provision of Security Surveillance System CCTV at Rourkela and Jharsuguda Railway stations at a cost of `0.420 Crore. Booking ofce with toilet complex for new entry at north side of Rourkela Station at a cost of `1 Crore. Water booth, Water Cooler at various platform of Rourkela and Rajgangpur Stations at a cost of `0.022 Crore. -
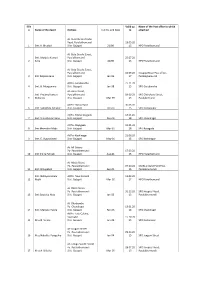
St Office to Which O Name of the Agent Address C.A No
Sl.N Valid up Name of the Post office to which o Name of the Agent Address C.A No. and Date to attached At- State Bank of India Road, Paralakhemundi 19.05.20 1 Smt. K. Bhudevi Dist- Gajapati 21/90 15 HPO Parakhemundi At- Bata Goudo Street, Smt. Manjula Kumari Paralakhemundi 25.07.20 2 Sahu Dist- Gajapati 42/90 15 HPO Parakhemundi At- Bata Goudo Street, Paralakhemundi 16.09.20 Hospital Road Post office, 3 Smt Kalpana Jena Dist- Gajapati Jan-96 17 Paralakjhemundi At/Po- Garabandha 27.11.20 4 Smt. B. Mangamma Dist- Gajapati Jan-98 15 SPO Garabandha At- Anka Street, Smt. Pratima Kumari Paralakhemundi 09.09.20 SPO Chitrakara Street, 5 Mohanty Dist- Gajapati Mar-99 15 Paralakemundi At/Po- Narayanpur 30.06.20 6 Smt. Sukuntala Sahukar Dist- Gajapati Feb-00 15 SPO Narayanpur At/Po- Mahendragada 13.01.20 7 Smt. Uma Kumari Sahu Dist- Gajapati Feb-04 18 SPO Chandragiri At/Po- Rayagada 19.01.20 8 Smt Meenakhi Majhi Dist- Gajapati Mar-01 18 SPO Rayagada At/Po- Kashinagar 21.06.20 9 Smt. E. Vijayaalaxmi Dist- Gajapati May-01 15 SPO Kashinagar At- MI Colony Po- Paralakhemundi 17.09.20 10 Smt Indira Patnaik Dist- Gajapati Aug-01 15 HPO Parakhemundi At- Matia Street Po- Paralakhemundi 07.10.20 Old Bus Stand Pst Office, 11 Smt. B Rupabati Dist- Gajapati Sep-01 15 Paralakhemundi Smt. Nakhyatramala At/Po- New Kerandi 12.09.20 12 Majhi Dist- Gajapati Mar-02 17 HPO Parakhemundi At- Elkela Street Po- Paralakhemundi 31.12.20 SPO Hospital Road, 13 Smt Sanjukta Hota Dist- Gajapati Jan-03 15 Paralakhemundi At- Dhadiambo Po- Chandragiri 13.01.20 14 Smt. -

UGC HUMAN RESOURCE DEVELOPMENT CENTRE SAMBALPUR UNIVERSITY 1. REFRESHER COURSE in CHEMISTRY (From 03.07.2014 to 23.07.2014)
UGC HUMAN RESOURCE DEVELOPMENT CENTRE SAMBALPUR UNIVERSITY 1. REFRESHER COURSE IN CHEMISTRY (From 03.07.2014 to 23.07.2014) Sl. Name, Designation & College Address of the participants No. 1. RAJESH KUMAR SAHOO Lecturer in Chemistry, B. J. B. Jr. College, Bhubaneswar- 751001 2. BHAGABAT BHUYAN Jr. Lecturer in Chemistry, F. M. Junior College, Balasore- 756001 3. SUJATA SAHU Lecturer in Chemistry, Betnoti College, Betnoti, Mayurbhanj-757025 4. BASANTA KUMAR BHOI Lecturer in Chemistry, Nilamani Mahavidyalaya, Rupsa, Balasore-756028 5. BISHNU CHARAN BEHERA Lecturer in Chemistry, M. G. Mahavidyalaya, Baisinga, Mayurbhanj- 757028 6. DEBA PRASANNA MOHANTY Lecturer in Chemistry, V. N. (Auto) College, Jajpur Road, Jajpur, 755019 7. DR. NILANCHALA PATEL Lecturer in Chemistry, Govt. (Auto) College, Rourkela, Sundargarh 8. RANJAN KUMAR PRADHAN Lecturer in Chemistry, V. Deb. College, Jeypore, Koraput- 764004 9. V. RAMESWAR RAJU Lecturer in Chemistry, Science College, Hinjilicut, Ganjam- 761102 10. SADASIBA MISHRA Lecturer in Chemistry, Panchayat College, Kalla, Deogarh- 768110 11. SADASHIBA NAYAK Lecturer in Chemistry, Saheed Memorial College, Manida, Mayurbhanj- 757020 12. DR. BRUNDABAN NAYAK Lecturer in Chemistry, People’s College, Buguda, Ganjam- 761118 13. DR. RAMAKRISHNA D. S. Lecturer in Chemistry, VSSUT, Burla- 768018 14. ASHOK PADHAN Lecturer in Chemistry, Attabira College, Attabira, Bargarh- 768027 15. DR. ARUNA KUMAR MISRA Lecturer in Chemistry, Rayagada (Auto) College, Rayagada 16. SUMANTA KUMAR PATEL, Lecturer in Chemistry, Kuchinda College, Kuchinda, Sambalpur 17. JYOTIRMAYEE PANIGRAHI Lecturer in Chemistry, Talcher (Auto) College, Talcher, Angul 18. SMITA RANI SAHOO Lecturer in Chemistry, Parjang College, Parjang 19. DR. MANORAMA SENAPATI Lecturer in Chemistry, N.S.B. Mahavidyalaya, Nuvapada, Ganjam 20. -

Standard Bid Document
ROURKELA SMART CITY LIMITED Procurement of Building Works on Turnkey Basis (Design & Execution) STANDARD BID DOCUMENT FOR REDEVELOPMENT OF PANPOSH MARKET AT ROURKELA (IN ABD AREA OF ROURKELA SMART CITY) ON TURNKEY BASIS GOVERNMENT OF ODISHA Redevelopment of Panposh Market at Rourkela (in ABD Area of Rourkela Smart City) BID ID NO 404(1)/Dt.06.07.2019. NATIONAL COMPETITIVE BIDDING (BUILDING WORKS ON TURN KEY BASIS WITH (ARCHITECTURAL DESIGN & EXECUTION) Redevelopment of Panposh Market at Rourkela (in ABD Area of Rourkela Smart City) on NAME OF WORK : Turnkey Basis (Architectural Planning with Design, Execution) PERIOD OF SALE OF BIDDING DOCUMENT : 08/07/2019 to 07/08/2019 TIME AND DATE OF PRE-BID CONFERENCE : DATE15/07/2019 TIME 11:00 HOURS LAST DATE AND TIME FOR RECEIPT OF BIDS DATE 07/08/2019 TIME 17:00 HOURS Online Mode LAST DATE AND TIME FOR RECEIPT OF BIDS DATE 13/08/2019 TIME 15:00 HOURS IN OFFLINE MODE *TIME AND DATE OF OPENING TECHNICAL BIDS : DATE13/08/2019 TIME 16:00 HOURS………. TIME AND DATE OF CONCEPT DRAWING : TO BE NOTIFIED LATER PRESENTATION *TIME AND DATE OF OPENING FINANCIAL BIDS : To be notified in the Web Portal of www..tendersodisha.gov.in PLACE OF OPENING OF BIDS : Conference Hall, Rourkela Smart City Limited OFFICER INVITING BIDS : Chief Executive Officer, Rourkela Smart City Limited 2 STANDARD BID DOCUMENT FOR REDEVELOPMENT OF PANPOSH MARKET AT ROURKELA (IN ABD AREA OF ROURKELA SMART CITY) ON TURNKEY BASIS INVITATION FOR BID (IFB) E-mail ID: [email protected] Tel. -(0661)-2500388 OFFICE OF THE CHIEF EXECUTIVE OFFICER, ROURKELA SMART CITY LIMITED, ROURKELA File No.