Perfluorinated Compounds in the Cape Fear Drainage Basin in North
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental Assessment of the Lower Cape Fear River System, 2013
Environmental Assessment of the Lower Cape Fear River System, 2013 By Michael A. Mallin, Matthew R. McIver and James F. Merritt August 2014 CMS Report No. 14-02 Center for Marine Science University of North Carolina Wilmington Wilmington, N.C. 28409 Executive Summary Multiparameter water sampling for the Lower Cape Fear River Program (LCFRP) has been ongoing since June 1995. Scientists from the University of North Carolina Wilmington’s (UNCW) Aquatic Ecology Laboratory perform the sampling effort. The LCFRP currently encompasses 33 water sampling stations throughout the lower Cape Fear, Black, and Northeast Cape Fear River watersheds. The LCFRP sampling program includes physical, chemical, and biological water quality measurements and analyses of the benthic and epibenthic macroinvertebrate communities, and has in the past included assessment of the fish communities. Principal conclusions of the UNCW researchers conducting these analyses are presented below, with emphasis on water quality of the period January - December 2013. The opinions expressed are those of UNCW scientists and do not necessarily reflect viewpoints of individual contributors to the Lower Cape Fear River Program. The mainstem lower Cape Fear River is a 6th order stream characterized by periodically turbid water containing moderate to high levels of inorganic nutrients. It is fed by two large 5th order blackwater rivers (the Black and Northeast Cape Fear Rivers) that have low levels of turbidity, but highly colored water with less inorganic nutrient content than the mainstem. While nutrients are reasonably high in the river channels, major algal blooms have until recently been rare because light is attenuated by water color or turbidity, and flushing is usually high (Ensign et al. -

Suspended Sediment and Nutrients in the Upper Cape Fear River Basin, North Carolina, 2002–04, with an Analysis of Temporal Changes, 1976–2004
Prepared in cooperation with the Upper Cape Fear River Basin Association Suspended Sediment and Nutrients in the Upper Cape Fear River Basin, North Carolina, 2002–04, with an Analysis of Temporal Changes, 1976–2004 Scientific Investigations Report 2005–5271 U.S. Department of the Interior U.S. Geological Survey Cover. Deep River spillway upstream from the U.S. highway 1 bridge in Chatham County, North Carolina (photograph by Ryan B. Rasmussen, U.S. Geological Survey). Suspended Sediment and Nutrients in the Upper Cape Fear River Basin, North Carolina, 2002–04, with an Analysis of Temporal Changes, 1976–2004 By Timothy B. Spruill, Phillip S. Jen, and Ryan B. Rasmussen Prepared in cooperation with the Upper Cape Fear River Basin Association Scientific Investigations Report 2005–5271 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior Gale A. Norton, Secretary U.S. Geological Survey P. Patrick Leahy, Acting Director U.S. Geological Survey, Reston, Virginia: 2006 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS--the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. -

The Trading Path and North Carolina
Journal of Backcountry Studies The Trading Path and North Carolina BY REBECCA TAFT FECHER Frontier is a subjective term, and one that has shifted and evolved over the years. North Carolina‟s piedmont and mountains originally were „frontier‟. The ever shifting frontier of North Carolina was explored cautiously, and with the help of the natives. In their explorations, and settlement of North Carolina, old native “trading paths” were utilized. Although some has been written about roads and paths into this area, most of the focus has been on the “Great Wagon Road”. A more fascinating look into the settlement of North Carolina‟s frontier is “the braided network of paths” and roads that make up what are referred to as the Great Trading Path.1 The label, in reality, referred to any number of different paths/roads within the state. This network allowed for the settlement of far flung areas in North Carolina, laying the ground work for where towns were formed, and providing a starting point for later roads throughout the state. Trade path routes were originally buffalo paths that then became Indian trails which were taken over by European explorers and settlers. Remnants of these old roads and trails have largely been forgotten as cars speed by, but can still be seen along many roads in this state. In many places, current roads overlay the centuries old paths. “The slender paths of aboriginal intercourse have been broadened and interwoven into the complex mazes of modern commercial lines”.2 Not only do these old roads represent our colonial beginnings, but they are a tangible part of our history that had a direct impact on where and how North Carolina was settled. -

A Case Study of Carolina Bays and Ditched Streams at Risk Under the Proposed WOTUS Definition
CAPE FEAR RIVER WATERSHED: A Case Study of Carolina Bays and Ditched Streams at Risk under the Proposed WOTUS Definition The Cape Fear River. Photo by Kemp Burdette The Cape Fear River Basin is North Carolina’s largest watershed, with an area of over 9,000 square miles. Major tributaries include the Deep River, the Haw River, the Northeast Cape Fear River, the Black River, and the South River. These rivers converge to form a thirty-mile-long estuary before flowing into the Atlantic Ocean at Cape Fear.1 The Cape Fear supplies water to some of the fastest growing counties in the United States;2 roughly one in five North Carolinians gets their drinking water from the Cape Fear, including residents of Greensboro, Fayetteville, and Wilmington.3 The Cape Fear Basin is a popular watershed for a variety of recreation activities. State parks along the river include Haw River State Park, Raven Rock State Park, and Carolina Beach State Park. The faster-flowing water of the upper basin is popular with paddlers, as are the slow meandering blackwater rivers and streams of the lower Cape Fear and estuary. Fishing is very popular; the Cape Fear supports a number of freshwater species, saltwater species, and even anadromous (migratory) species like the endangered sturgeon, striped bass, and shad. Cape Fear River Watershed: Case Study Page 2 of 8 The Cape Fear is North Carolina’s most ecologically diverse watershed; the Lower Cape Fear is notable because it is part of a biodiversity “hotspot,” recording the largest degree of biodiversity on the eastern seaboard of the United States. -

Cape Fear River Blueprint
Lower Cape Fear River Blueprint Since 1982, the North Carolina Coastal Federation has worked with residents and visitors of North Carolina to protect and restore the coast, including participating in many issues and projects within the Cape Fear region. The Lower Cape Fear River provides critical coastal and riverine habitat, storm and flood protection and commercial and recreational fishery resources. It is also a popular recreational destination and an economic driver for the state’s southeastern region, and it embodies a rich historic and cultural heritage. The river also serves as an important source of drinking water for many coastal communities, including the City of Wilmington. Today, the lower river needs improved environmental safeguards, a clear vision for compatible and sustainable economic development. Further, effective leadership is needed to address existing issues of pollution and habitat loss and prevent projects that threaten the health of the community and the river. The Lower Cape Fear River is continually affected by both local and upstream actions. The continued degradation of these essential functions threatens the well-being of all within the community. The Coastal Federation continues to address these issues by actively engaging with local governments, state and federal agencies and other entities. Our board of directors recognizes these needs and adopted the following goals as part of a three-year, organizationwide initiative: Advocate for compatible industrial development in the coastal zone; Restore coastal habitats and protect water quality; Improve our economy through coastal restoration. The Lower Cape Fear River Blueprint (The Blueprint) is a collaborative effort to focus on the river’s estuarine and riverine natural resources. -

Environmental Assessment of the Lower Cape Fear River System, 2015
Environmental Assessment of the Lower Cape Fear River System, 2015 By Michael A. Mallin, Matthew R. McIver and James F. Merritt November 2016 CMS Report No. 16-02 Center for Marine Science University of North Carolina Wilmington Wilmington, N.C. 28409 Executive Summary Multiparameter water sampling for the Lower Cape Fear River Program (LCFRP) http://www.uncw.edu/cms/aelab/LCFRP/index.htm, has been ongoing since June 1995. Scientists from the University of North Carolina Wilmington’s (UNCW) Aquatic Ecology Laboratory perform the sampling effort. The LCFRP currently encompasses 33 water sampling stations throughout the lower Cape Fear, Black, and Northeast Cape Fear River watersheds. The LCFRP sampling program includes physical, chemical, and biological water quality measurements and analyses of the benthic and epibenthic macroinvertebrate communities, and has in the past included assessment of the fish communities. Principal conclusions of the UNCW researchers conducting these analyses are presented below, with emphasis on water quality of the period January - December 2015. The opinions expressed are those of UNCW scientists and do not necessarily reflect viewpoints of individual contributors to the Lower Cape Fear River Program. The mainstem lower Cape Fear River is a 6th order stream characterized by periodically turbid water containing moderate to high levels of inorganic nutrients. It is fed by two large 5th order blackwater rivers (the Black and Northeast Cape Fear Rivers) that have low levels of turbidity, but highly colored water with less inorganic nutrient content than the mainstem. While nutrients are reasonably high in the river channels, major algal blooms have until recently been rare because light is attenuated by water color or turbidity, and flushing is usually high (Ensign et al. -

Native Americans in the Cape Fear, by Dr. Jan Davidson
Native Americans in the Cape Fear, By Dr. Jan Davidson Archaeologists believe that Native Americans have lived in what is now the state of North Carolina for more than 13,000 years. These first inhabitants, now called Paleo-Indians by experts, were likely descended from people who came over a then-existing land bridge from Asia.1 Evidence had been found at Town Creek Mound that suggests Indians lived there as early as 11000 B.C.E. Work at another major North Carolinian Paleo-Indian where Indian artifacts have been found in layers of the soil, puts Native Americans on that land before 8000 B.C.E. That site, in North Carolina’s Uwharrie Mountains, near Badin, became an important source of stone that Paleo and Archaic period Indians made into tools such as spears.2 It is harder to know when the first people arrived in the lower Cape Fear. The coastal archaeological record is not as rich as it is in some other regions. In the Paleo-Indian period around 12000 B.C.E., the coast was about 60 miles further out to sea than it is today. So land where Indians might have lived is buried under water. Furthermore, the coastal Cape Fear region’s sandy soils don’t provide a lot of stone for making tools, and stone implements are one of the major ways that archeologists have to trace and track where and when Indians lived before 2000 B.C.E.3 These challenges may help explain why no one has yet found any definitive evidence that Indians were in New Hanover County before 8000 B.C.E.4 We may never know if there were indigenous people here before the Archaic period began in approximately 8000 B.C.E. -
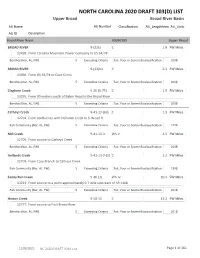
NORTH CAROLINA 2020 DRAFT 303(D) LIST Upper Broad Broad River Basin
NORTH CAROLINA 2020 DRAFT 303(D) LIST Upper Broad Broad River Basin AU Name AU Number Classification AU_LengthArea AU_Units AU ID Description Broad River Basin 03050105 Upper Broad BROAD RIVER 9-(22)a C 1.8 FW Miles 12499 From Carolina Mountain Power Company to US 64/74 Benthos (Nar, AL, FW) 5 Exceeding Criteria Fair, Poor or Severe Bioclassification 2008 BROAD RIVER 9-(22)b1 C 6.3 FW Miles 13396 From US 64/74 to Cove Creek Benthos (Nar, AL, FW) 5 Exceeding Criteria Fair, Poor or Severe Bioclassification 2008 Cleghorn Creek 9-26-(0.75) C 1.5 FW Miles 13295 From 90 meters south of Baber Road to the Broad River Benthos (Nar, AL, FW) 5 Exceeding Criteria Fair, Poor or Severe Bioclassification 2008 Catheys Creek 9-41-13-(6)b C 1.9 FW Miles 12701 From confluence with Hollands Creek to S. Broad R. Fish Community (Nar, AL, FW) 5 Exceeding Criteria Fair, Poor or Severe Bioclassification 1998 Mill Creek 9-41-13-3 WS-V 4.5 FW Miles 12705 From source to Catheys Creek Benthos (Nar, AL, FW) 5 Exceeding Criteria Fair, Poor or Severe Bioclassification 2008 Hollands Creek 9-41-13-7-(3) C 2.2 FW Miles 12709 From Case Branch to Catheys Creek Fish Community (Nar, AL, FW) 5 Exceeding Criteria Fair, Poor or Severe Bioclassification 1998 Sandy Run Creek 9-46-(1) WS-IV 10.4 FW Miles 13221 From source to a point approximately 0.7 mile upstream of SR 1168 Fish Community (Nar, AL, FW) 5 Exceeding Criteria Fair, Poor or Severe Bioclassification 2018 Hinton Creek 9-50-15 C 13.2 FW Miles 12777 From source to First Broad River Benthos (Nar, AL, FW) 5 Exceeding Criteria -

Emerging Per- and Polyfluoroalkyl Substances: the North Carolina Experience
Emerging Per- and Polyfluoroalkyl Substances: The North Carolina Experience Zack Moore, MD, MPH State Epidemiologist Division of Public Health, N.C. DHHS Outline • Timeline of GenX response and scope of issue • Public health responses • Challenges and data gaps • Ongoing and future activities Wilmington, North Carolina Timeline of Response to GenX in Drinking Water • June 2017 −News coverage brings GenX to the forefront −N.C. DHHS contacted by local health departments 4 What is GenX? • Replacement chemical for PFOA • Perfluoroalkyl Ether Carboxylic Acid (PFECA) • Manufactured since 2009 • Byproduct of other manufacturing processes since ~1980 Scope of Problem – Surface water sourced public water • Hundreds of thousands public water customers affected by GenX and other PFAS in Cape Fear River Sun et al 2016 Timeline of Response to GenX in Drinking Water • June 2017 • August 2017 −News coverage brings GenX to −GenX detected in monitoring the forefront wells on-site −N.C. DHHS contacted by local • September 2017 health departments −Private well sampling near the −N.C. DHHS issues initial drinking facility begins water assessment −Chemours stops discharge of GenX to Cape Fear River • July 2017 −N.C. DHHS updates assessment and issues a provisional health goal of 140 ng/L (ppt) Scope of Problem – Groundwater sourced private wells • Hundreds of parcels with private wells near the facility potentially impacted by GenX and other PFAS • Phase 1 & 2 GenX results −Blue: ND −Green: 11-100 ng/L −Yellow: 101-140 ng/L −Orange: 141-420 ng/L −Red: >420 ng/L N.C. DEQ Scope of Problem – Groundwater sourced private wells • Sampling will continue until full extent of contamination is determined −Dark blue: phase 1 −Orange: phase 2 −Light blue/yellow: phase 3 −Fuchsia: phase 4 N.C. -

Genx, Environmental Justice, and North Carolina's Legacy of Pollution
GenX, Environmental Justice, and North Carolina’s Legacy of Pollution Christine Billings Nicholas Krebs Jatu F. Nugrohorukmi Cameron Smith Mackenzie Todd December 8, 2017 Department of Public and International Affairs Leutze Hall, University of North Carolina Wilmington Wilmington, NC 28403 GenX, Environmental Justice, and North Carolina’s Legacy of Pollution Final Project Fall Semester 2017 Prepared For Dr. Weinkle’s MCOP 595 Prepared By University of North Carolina at Wilmington Coastal and Ocean Policy Program The cover page pictures were provided by the EPA and StarNews Online. Maps throughout this paper were created using ArcGIS software by Esri. ArcGIS and ArcMap are the intellectual property of Esri and are used herein under license. Copyright © Esri. All Rights Reserved. For more information about Esri software, please visit www.esri.com. Acknowledgements The authors would like to thank Dr. Rob Hart of UNC Wilmington Department of History, Dr. Larry Cahoon of UNC Wilmington Department of Marine Biology, and Kemp Burdette of Cape Fear River Watch. i Table of Contents Acknowledgements ........................................................................................................................ i Introduction ................................................................................................................................... 1 Literature Review ......................................................................................................................... 4 Methods ......................................................................................................................................... -

Coastal Resilience Assessment of the Cape Fear Watershed
PILOT: Coastal Resilience Assessment of the Cape Fear Watershed i IMPORTANT INFORMATION/DISCLAIMER: This report represents a Regional Coastal Resilience Assessment that can be used to identify places on the landscape for resilience-building efforts and conservation actions through understanding coastal flood threats, the exposure of populations and infrastructure have to those threats, and the presence of suitable fish and wildlife habitat. As with all remotely sensed or publicly available data, all features should be verified with a site visit, as the locations of suitable landscapes or areas containing flood hazards and community assets are approximate. The data, maps, and analysis provided should be used only as a screening-level resource to support management decisions. This report should be used strictly as a planning reference tool and not for permitting or other legal purposes. The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the authors and should not be interpreted as representing the opinions or policies of the U.S. Government, or the National Fish and Wildlife Foundation’s partners. Mention of trade names or commercial products does not constitute their endorsement by the U.S. Government or the National Fish and Wildlife Foundation or its funding sources. NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION DISCLAIMER: The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the author(s) and do not necessarily reflect those of NOAA or the Department of Commerce. U.S. ARMY CORPS OF ENGINEERS DISCLAIMER: NFWF’s assessment methodology focuses on identifying and ranking Resilience Hubs, or undeveloped areas of open space. -

The Influence of Tidal Inlet Migration and Closure on Barrier Planform Changes: Federal Beach, Nc
THE INFLUENCE OF TIDAL INLET MIGRATION AND CLOSURE ON BARRIER PLANFORM CHANGES: FEDERAL BEACH, NC Emerson G. Hasbrouck A Thesis submitted to the University of North Carolina Wilmington in Partial Fulfillment of the Requirements for the Degree of Master of Science Department of Geography and Geology University of North Carolina Wilmington 2007 Approved by Advisory Committee _____________________________ _____________________________ _______________________________ Chair Accepted by _____________________________ Dean, Graduate School This thesis has been prepared in a style and format consistent with The Journal of Coastal Research ii TABLE OF CONTENTS ABSTRACT.........................................................................................................................v DEDICATION.................................................................................................................. vii LIST OF TABLES........................................................................................................... viii LIST OF FIGURES ........................................................................................................... ix INTRODUCTION ...............................................................................................................1 STUDY AREA ....................................................................................................................4 Physical Setting....................................................................................................................4