University of Michigan University Library
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Houghton Lake Roscommon County May 22, 2012
Michigan Dept. of Natural Resources 2012-141 Status of the Fishery Resource Report Page 1 Houghton Lake Roscommon County May 22, 2012 Richard P. O'Neal, Fisheries Biologist, Muskegon Environment Houghton Lake is located in the central portion of the Lower Peninsula of Michigan in Roscommon County (Figure 1). The lake is part of the headwaters of the Muskegon River Watershed along with Higgins Lake and the Cut River that connects the two lakes. Houghton Lake is the largest inland Lake in Michigan with an area of 20,075 surface acres (Breck 2004). The maximum depth of Houghton Lake is 22 feet, and the majority of the lake is shallow with an average depth of 8.4 feet and an estimated total volume of 165,072 acre-feet. Houghton Lake has several small tributary streams, including Backus, Denton, and Knappen creeks. The primary upstream flow comes from Backus Creek, which flows through a series of reservoirs about 6 miles upstream from Houghton Lake. Denton Creek also flows through a reservoir about 1 mile upstream. The Cut River, a tributary of Backus Creek, connects Houghton Lake with 238-acre Marl Lake and 9,600-acre Higgins Lake. The distance between Houghton and Higgins lakes is about 10 stream miles. Downstream from Houghton Lake, the Muskegon River initially flows to the northwest but eventually curves to the southwest and flows for 212 miles to Lake Michigan (O'Neal 1997). Human development within the watershed of Houghton Lake has significantly altered fisheries habitat within and around the lake. Affects of development have been typical of those reported for other north temperate lakes (Christensen et al. -

2019/2020 Winter Newsletter
Winter 2020 Higgins Lake Property Owners Association AuSable River Center, 211 North Main Street Roscommon, MI 48653 Website: www.hlpoa.org Email: [email protected] Ph.: (989) 275-9181 Fax: (989) 275-9182 Office Hours: M, W & Th from 9 am - 2:00 pm. To Protect, Preserve and Enhance the Quality of Higgins Lake and Its Surrounding Watershed President’s Message In this edition: HLPOA begins 2020 with a celebration: it has been 85 years President’s Message since the founding of HLPOA, the premiere lake association Membership Renewal in the state. HLPOA stands alone as the longest-serving, DNR 2019 Invasive primary force striving to preserve the beauty and quality of Species Program Higgins Lake. Articles of Association were filed in July 1935, signed by four friends. The families of two signers, DNR 2020 Aquatic Arthur A. Meyer and Paul H. Bruske, still enjoy our Invasive Species Plan wonderful lake. We would like to learn more about our New Years Resolution founding. Please let us know if you have any information on the other two families of Sewer Project Update signers, Lawrence B. Benkert and Curt P. Schneider, which would help in contacting Science Corner them. Articles of Incorporation, with two additional signers, were filed in January 1940. Any information on them, Howard W. Peirce, M.D., and Alan H. Pearson, would Plastics be greatly appreciated, too. AMVETS lawsuit Cut River History We hope you enjoy reading this newsletter and stay up to date with activities of Resources HLPOA during the winter months. You’ll notice a couple articles of interest written by Sincere Thanks fellow members. -
Roscommon County Community Resource Guide
Roscommon County Community Resource Guide Resources assembled by: Roscommon County – Community Resources 2021 Available at http://www.mclaren.org/mclaren-physician-partners/resources.aspx Roscommon County 2021 Community Resource Guide [A guide to services in your community] [TABLE OF CONTENTS] Abuse & Protective Services........................................................................ [1] Adult Aging Services ................................................................................... [1] Animal Support ........................................................................................... [2] County & City Resources ............................................................................. [2] Disability Resources .................................................................................... [2] Education & Training .................................................................................. [2] Emergency & Disaster Services ................................................................... [3] Employment Assistance .............................................................................. [3] Financial Assistance .................................................................................... [3] Tax Assistance ............................................................................................ [3] Fitness, Recreation & Rehabilitation ........................................................ [3-4] Food, Clothing & Household Assistance ..................................................... -
Designated Trout Lakes and Streams
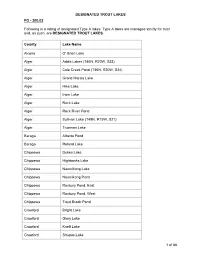
DESIGNATED TROUT LAKES FO - 200.02 Following is a listing of designated Type A lakes. Type A lakes are managed strictly for trout and, as such, are DESIGNATED TROUT LAKES. County Lake Name Alcona O' Brien Lake Alger Addis Lakes (T46N, R20W, S33) Alger Cole Creek Pond (T46N, R20W, S24) Alger Grand Marais Lake Alger Hike Lake Alger Irwin Lake Alger Rock Lake Alger Rock River Pond Alger Sullivan Lake (T49N, R15W, S21) Alger Trueman Lake Baraga Alberta Pond Baraga Roland Lake Chippewa Dukes Lake Chippewa Highbanks Lake Chippewa Naomikong Lake Chippewa Naomikong Pond Chippewa Roxbury Pond, East Chippewa Roxbury Pond, West Chippewa Trout Brook Pond Crawford Bright Lake Crawford Glory Lake Crawford Kneff Lake Crawford Shupac Lake 1 of 86 DESIGNATED TROUT LAKES County Lake Name Delta Bear Lake Delta Carr Lake (T43N, R18W, S36) Delta Carr Ponds (T43N, R18W, S26) Delta Kilpecker Pond (T43N, R18W, S11) Delta Norway Lake Delta Section 1 Pond Delta Square Lake Delta Wintergreen Lake (T43N, R18W, S36) Delta Zigmaul Pond Gogebic Castle Lake Gogebic Cornelia Lake Gogebic Mishike Lake Gogebic Plymouth Lake Houghton Penegor Lake Iron Deadman’s Lk (T41N, R32W, S5 & 8) Iron Fortune Pond (T43N, R33W, S25) Iron Hannah-Webb Lake Iron Killdeer Lake Iron Madelyn Lake Iron Skyline Lake Iron Spree Lake Isabella Blanchard Pond Keweenaw Manganese Lake Keweenaw No Name Pond (T57N, R31W, S8) Luce Bennett Springs Lake Luce Brockies Pond (T46N, R11W, S1) 2 of 86 DESIGNATED TROUT LAKES County Lake Name Luce Buckies Pond (T46N, R11W, S1) Luce Dairy Lake Luce Dillingham -

Muskegon River Fisheries Management Summaries 02232017
Department of Natural Resources, Fisheries Division Muskegon River Fisheries Management Summaries February 23, 2017 Richard P. O’Neal The Muskegon River begins in north-central Lower Michigan, flowing from Higgins Lake and Houghton Lakes, southwesterly to the City of Muskegon and discharging into central Lake Michigan. The river is 212 miles long and drops 575 feet in elevation between its headwater and mouth (O’Neal 1997). The watershed incorporates over 2,350 square miles of land and approximately 94 tributaries flow directly into the mainstem. The original fish community of the river system was composed of 97 species, and there have been a number of species that colonized or were introduced. The most recent fish community surveys were conducted on Higgins Lake (unpublished 2011), Houghton Lake (O’Neal 2011), the river and large impoundments (O’Neal 2002), and Muskegon Lake (unpublished 2008). The Muskegon River Watershed Assessment provides substantial background on the physical characteristics of the river (O’Neal 1997), along with Wiley et al. (2009). The hydrology of the river is intermediate between coldwater and warmwater Michigan streams. Water temperature data and fisheries surveys verify the coolwater nature of this river (O’Neal 2001; O’Neal 2011). The predominant species of fish found in the river are suckers, but there are many sport fish also present in the river as well as the lakes. The lower river (from Croton Dam to Muskegon Lake), Muskegon Lake, Houghton Lake, and Higgins Lake provide some of the best fishing in Michigan. These summaries are intended to highlight the existing fisheries in the river system, and provide brief summaries of recent studies conducted on important fish species along with needed research. -

Michigan Forest History
-Forest Basics- Michigan Forest History Adapted from the on-line Teachers Guide http://mff.dsisd.net 1 0 MICHIGAN FOREST HISTORY www.dsisd.k12.mi.us/mff BEGINNING MODERN ICE PRESETTLEMENT LOGGING OF FOREST AGE TIMES ERA CONSERVATION MANAGEMENT Note: This timeline is NOT to scale. The left side represents many more years than the right. The right side shows more years because there were more things happening . and there still are more things happening in the forest today! Michigan has a colorful forest history, similar to that of Wisconsin and Minnesota. After the retreat of the glaciers, vegetation gradually moved back into Michigan. Some tree species returned centuries before other tree species. Our forests are ever-changing from the effects of climate, nature, and the influences of human beings. American Indians changed the forest in many ways. When people began logging the forest in the middle 1800s, the forest at that time was in a condition useful to our growing nation. Our forests continue to be great natural resources, both for wood production as well as the many other benefits we receive from the Great Forests of the Great Lakes State! 1 MICHIGAN FORESTS DURING THE ICE AGE It's pretty easy to imagine what forests looked like during the Ice Age. There were no forests! At least not in what we now call Michigan. In fact, there wasn't much in the way of living things at all. Michigan was covered with as much as a mile of ice! So, where were all the trees and other living things that make up our forests today? Glaciers cooled nearby areas so that northern species could live farther and farther south as the glaciers advanced. -

Dam-Study-Uofm2016.Pdf
Final Report to the Muskegon River Watershed Assembly Name of Study: Ecohydrologic Evaluation of Removing the Higgins Lake-Level Control Structure. Prime Contractor: Muskegon River Watershed Assembly Study Job D.6 Prepare Habitat Models to Examine Fishery-Related Impacts University of Michigan Subcontract. Michael J Wiley and Andrew J Layman. A. Problem: Higgins Lake, in Roscommon County, has a controversial level of shore erosion which has been attributed to high water caused by an old lake-level control structure (dam) at the junction of the lake and the Cut River. The erosion has been severe enough to concern the Higgins Lake Property Owners Association but effects of the erosion on the lake bottom, surrounding vegetation, animal species, and neighboring aquatic habitats have had little study since the dam’s construction in 1936. This is despite the fact that the inter- connected Higgins Lake-Cut River-Houghton Lake system comprises the headwaters of the Muskegon River and supports a major inland recreational fishery for Yellow Perch, Smallmouth Bass, Walleye, Lake Trout, Rainbow Trout, Lake Whitefish, Rainbow Smelt, and a number of other species (O’Neal 1997, 2003). The number of angler hours measured during a one year period (2001 – 2002) was 250,962 hours on Higgins Lake and 499,048 hours on Houghton Lake. No data was collected for the Cut but the angler use is relatively high for a smaller river system. The economic value of this combined fishery to the local economy is estimated by MDNR to exceed $6.9 million annually. DNR Fisheries Division stocks Higgins Lake every year with 75,000 trout including lake trout, rainbow trout and brown trout at an annual cost of approximately $75,000. -

2021 Fishing Guide
2021 Michigan Fishing Guide Rules apply from April 1, 2021 - March 31, 2022 Get the digital guide at Michigan.gov/DNRDigests MICHIGAN FISHING LICENSE INFORMATION & REQUIREMENTS License Items & Fees Purchase your fshing license (and more) online at Michigan.gov/DNRLicenses. Item Type Fee DNR Sportcard $1 All -Species Fishing Licenses Resident Annual $26* Nonresident Annual $76* Senior Annual (Residents 65 or older or Residents who are legally blind – see p. 6) $11* Daily Fish (Resident or Nonresident) $10/day Angler can buy from 1 to 6 consecutive days Voluntary Youth (16 and under) $2* Combo Hunt/Fish Licenses (Base, Annual Fishing, 2 Deer) Hunt/Fish Resident $76* Hunt/Fish Senior Resident $43* Hunt/Fish Nonresident $266* Lost Licenses Lost your license? See the gray box below for instructions on getting a replacement. Fishing license fees are waived for Michigan residents who are veterans with 100% disability or active-duty military (see p. 6). All fshing licenses are good for all species allowed for harvest as indicated in this Fishing Guide. A $1 surcharge is included in the combo hunt and fsh licenses, resident annual, nonresident annual, and senior annual fshing licenses, as noted with the asterisk (*). Revenue generated from these funds will be used to educate the public on the benefts of hunting, fshing and trapping in Michigan, and the impact of these activities on the conservation, preservation and management of the state’s natural resources in accordance with statute. Learn more at Michigan.gov/DNR. Who needs a Michigan fshing license? Any adult actively assisting a minor must have A license is required when targeting fsh, a fshing license. -

Recreation Plan Steering Committee Sue Jock-Roscommon Metropolitan Recreation Authority, Chair Connie Allen-Secretary, Higgins Twp
HIGGINS TOWNSHIP VILLAGE OF ROSCOMMON PARK, RECREATION AND OPEN SPACE MASTER PLAN 2018-2022 COMMUNITY ADVISERY Higgins Township Board Bill Curnalia-Supervisor Cheri Sullivan-Treasurer LeAnna Goodrich-Clerk Trustee-Ron Peters Trustee-Tom Jennette Village Council Dan Fishel-President Marc McKee-President Pro-tempore Heather Roemer Mike Miller Jan Deloge Brian Ludwig Dan Scow Recreation Plan Steering Committee Sue Jock-Roscommon Metropolitan Recreation Authority, Chair Connie Allen-Secretary, Higgins Twp. Diane Lippert-COOR Intermediate School District Julie Crick-Natural Resource Educator-MSUe John Rosczyk-Village Manager Bill Curnalia-Higgins Township Supervisor Ron Alden-Higgins Township Treasurer Nancy Smitz-Community Member Roscommon Metropolitan Recreation Authority Board Sue Jock Connie Allen Eric Hart Karrie Williams Cheri Hutek Derek Smitz TABLE OF CONTENTS Introduction and Planning Process Chapter 1 Community Description 5 Chapter 2 Administrative Structure 8 Chapter 3 Recreation and Resource Inventories 10 DNR Recreation Grant Inventory 20 Chapter 4 Evaluation of 2013-2017 Plan 21 Chapter 5 Description of Planning Process 23 Chapter 6 Planning Methods 25 Chapter 7 Description of the Public Input Process 26 Chapter 8 Goals and Objectives 28 Chapter 9 Action Program 32 APPENDIX Resource Inventory 36 Community Questionnaire 37 Recreation Related Articles from Newspapers 60 Recreation Plan Availability Public Notice 66 Chamber of Commerce Presentation Minutes 67 Recreation Committee Presentation Minutes 68 Notice of Planning Commission Public Hearing 69 Planning Commission Approval Minutes 70 Planning Commission Approval Resolution 71 Recreation Committee Approval Minutes 72 Higgins Township Approval Resolution 73 Village Council Approval Resolution 74 Submittal Letter to Roscommon County Planning Commission 75 Submittal Letter to Eastern Michigan Council of Governments 76 Recreation Plan Certification Checklist 77 INTRODUCTION A driving economic impact for today’s communities is access to recreation. -
Roscommon County Planning Commission
ROSCOMMON COUNTY PLANNING COMMISSION ROSCOMMON COUNTY MASTER PLAN FOR LAND USE GOALS AND OBJECTIVES FOR DEVELOPMENT AND GROWTH MANAGEMENT July 19, 2010 ROSCOMMON COUNTY BOARD Ed Nellist, Chairman District 1 Ken Melvin District 2 Bob Schneider, Vice Chairman District 3 Marc J. Milburn District 4 Pam Stephan District 5 ROSCOMMON COUNTY PLANNING COMMISSION Mike Wahl Chairman Timothy Grahl Vice Chairperson Barbara Stauffer Secretary Harold Williams Member Bob Schneider Commissioner/Member Candi Stroh Member Carl Seils Member CONSULTANTS Don Hamilton, A.I.C.P. Scott Bell, Assistant Planner & GIS Specialist Clare West Branch Roscommon County, Michigan i Roscommon County Master Plan Table of Contents Section Page 1. Introduction ..............................................................................1 2. Background A. Regional History....................................................................3 B Geography..............................................................................10 C. Land Use & Development Patterns........................................20 D. Demographic Trends..............................................................26 E. Community Facilities & Services ..........................................29 F. Transportation & Utilities......................................................38 G. Economic Trends & Profile ...................................................45 H. Attractions & Activities .........................................................55 3. Future Land Use A. The Great Recession ..............................................................59 -

Additional Questions-And-Answers from April 30 DNR Live: FISH Event
Additional Questions & Answers from April 30 DNR Live: FISH Event The information below reflects questions and answers the DNR was unable to answer during its April 30 DNR Live: FISH event. 1. If I have something I want to share with the DNR’s Fisheries Division, how can I make my voice heard? There are several ways you can get in touch with the DNR. One way would be to contact your local DNR office (visit any page at www.michigan.gov/dnr and click on “Contact DNR” at the top of the page to find the office closest to you). Another way would be to send an email to [email protected]. 2. Why does the Lake Erie walleye possession limit change each year? Michigan's daily creel limit for walleyes on Lake Erie is based on its share of the Total Allowable Catch (TAC) for the lake, which is determined each year by the Lake Erie Committee under the guidance of the Great Lakes Fishery Commission. The TAC is generally based on overall abundance of walleyes; the committee establishes quotas for each jurisdiction based on the percentage of habitat for adult walleyes in each jurisdiction's waters of the lake. The daily limit is based on a formula that projects how many walleyes anglers can keep but still remain within the quota. 3. Are there any plans to create additional spawning areas on the Detroit and St. Clair Rivers? There are plans to create more spawning areas. In the last few years two spawning areas have been created in the Detroit River, off Belle Isle and Fighting Island. -

Effects of Residential Development on the Water Quality of Higgins Lake, Michigan 1995-99
U.S. Department of the Interior U.S. Geological Survey Effects of Residential Development on the Water Quality of Higgins Lake, Michigan 1995-99 Water-Resources Investigations Report 01-4055 In cooperation with Gerrish and Lyon Townships, Roscommon County, Michigan, Higgins Lake Property Association and Higgins Lake Foundation Effects of Residential Development on the Water Quality of Higgins Lake, Michigan 1995-99 By Russel J. Minnerick U.S. Geological Survey Water-Resources Investigations Report 01-4055 Lansing, Michigan 2001 U.S. DEPARTMENT OF THE INTERIOR GALE A. NORTON, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director For additional information write: Copies of this report can purchased from: District Chief U.S.Geological Survey U.S. Geological Survey Branch of Information Services 6520 Mercantile Way, Ste. 5 Box 25286 Lansing, MI 48911-5991 Denver, CO 80225-0286 CONTENTS ���� Abstract . 1 Introduction . 1 Purpose and scope . 1 Description of the study area . 3 Study design . 3 Acknowledgments . 4 Study Methods. 5 Water-quality sites . 6 Water elevations . 9 Residential density . 9 Water-quality characteristics . 10 Shallow ground water. 10 Near-shore lake water. 12 Inlets . 12 Deep basins. 15 Effects of residential development on water quality . 19 Summary and conclusions . 26 References cited. 27 FIGURES 1. Map showing study area and location of data collection sites at and near Higgins Lake, Roscommon, Michigan . 2 FIGURES 2-9 Graphs showing: 2. Relation between total phosphorus and E. Coli in ground water at sampling site 33, Higgins Lake . 12 3. Temperature and oxygen profiles for Higgins Lake, June 1996 to September 1996 . 16 4.