Candidatus Phytoplasma Meliae’ (Isolate Chtyxiii)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cambridge Companion to Greek Mythology (2007)
P1: JzG 9780521845205pre CUFX147/Woodard 978 0521845205 Printer: cupusbw July 28, 2007 1:25 The Cambridge Companion to GREEK MYTHOLOGY S The Cambridge Companion to Greek Mythology presents a comprehensive and integrated treatment of ancient Greek mythic tradition. Divided into three sections, the work consists of sixteen original articles authored by an ensemble of some of the world’s most distinguished scholars of classical mythology. Part I provides readers with an examination of the forms and uses of myth in Greek oral and written literature from the epic poetry of the eighth century BC to the mythographic catalogs of the early centuries AD. Part II looks at the relationship between myth, religion, art, and politics among the Greeks and at the Roman appropriation of Greek mythic tradition. The reception of Greek myth from the Middle Ages to modernity, in literature, feminist scholarship, and cinema, rounds out the work in Part III. The Cambridge Companion to Greek Mythology is a unique resource that will be of interest and value not only to undergraduate and graduate students and professional scholars, but also to anyone interested in the myths of the ancient Greeks and their impact on western tradition. Roger D. Woodard is the Andrew V.V.Raymond Professor of the Clas- sics and Professor of Linguistics at the University of Buffalo (The State University of New York).He has taught in the United States and Europe and is the author of a number of books on myth and ancient civiliza- tion, most recently Indo-European Sacred Space: Vedic and Roman Cult. Dr. -

Extracts on Eggs, Nymphs and Adult Red Spider Mites, Tetranychus Spp. (Acari: Tetranychidae) on Tomatoes
African Journal of Agricultural Research Vol.8(8), pp. 695-700, 8 March, 2013 Available online at http://www.academicjournals.org/AJAR DOI: 10.5897/AJAR12.2143 ISSN 1991-637X ©2013 Academic Journals Full Length Research Paper Efficacy of Syringa (Melia Azedarach L.) extracts on eggs, nymphs and adult red spider mites, Tetranychus spp. (Acari: Tetranychidae) on tomatoes Mwandila N. J. K.1, J. Olivier1*, D. Munthali2 and D. Visser3 1Department of Environmental Sciences, University of South Africa (UNISA), Florida 1710, South Africa. 2Department of Crop Science and Production, Botswana College of Agriculture, Private Bag 0027, Gaborone, Botswana. 3ARC-Roodeplaat Vegetable and Ornamental Plant Institute (VOPI), Private Bag X293, Pretoria 0001, South Africa. Accepted 18 February, 2013 This study evaluated the effect of Syringa (Melia azedarach) fruit and seed extracts (SSE) on red spider mite (Tetranychus spp.) eggs, nymphs and adults. Bioassay investigations were carried at the Vegetable and Ornamental Plant Institute (VOPI) outside Pretoria in South Africa using different concentrations (0.1, 1, 10, 20, 50, 75 and 100%) of SSE. Mortalities were measured at 24, 48 and 72 h after treatment and compared to the effects of the synthetic acaricides: Abamectin, chlorfenapyr and protenofos. A completely randomized design (CRD) was used with 12 treatments. Analysis of variance (ANOVA) was used to test for effects of treatments. Differences in treatment means were identified using Fisher’s protected t-test least significant difference (LSD) at the 1% level of significance. Data were analysed using the statistical program GenStat (2003). The result of the analyses revealed that the efficacy of SSE and commercial synthetic acaricides increased with exposure time. -

'Jaws'. In: Hunter, IQ and Melia, Matthew, (Eds.) the 'Jaws' Book : New Perspectives on the Classic Summer Blockbuster
This is the accepted manuscript version of Melia, Matthew [Author] (2020) Relocating the western in 'Jaws'. In: Hunter, IQ and Melia, Matthew, (eds.) The 'Jaws' book : new perspectives on the classic summer blockbuster. London, U.K. For more details see: https://www.bloomsbury.com/uk/the-jaws-book-9781501347528/ 12 Relocating the Western in Jaws Matthew Melia Introduction During the Jaws 40th Anniversary Symposium1 Carl Gottlieb, the film’s screenwriter, refuted the suggestion that Jaws was a ‘Revisionist’ or ‘Post’ Western, and claimed that the influence of the Western genre had not entered the screenwriting or production processes. Yet the Western is such a ubiquitous presence in American visual culture that its narratives, tropes, style and forms can be broadly transposed across a variety of non-Western genre films, including Jaws. Star Wars (1977), for instance, a film with which Jaws shares a similar intermedial cultural position between the Hollywood Renaissance and the New Blockbuster, was a ‘Western movie set in Outer Space’.2 Matthew Carter has noted the ubiquitous presence of the frontier mythos in US popular culture and how contemporary ‘film scholars have recently taken account of the “migration” of the themes of frontier mythology from the Western into numerous other Hollywood genres’.3 This chapter will not claim that Jaws is a Western, but that the Western is a distinct yet largely unrecognised part of its extensive cross-generic hybridity. Gottlieb has admitted the influence of the ‘Sensorama’ pictures of proto-exploitation auteur William Castle (the shocking appearance of Ben Gardner’s head is testament to this) as well as The Thing from Another World (1951),4 while Spielberg suggested that they were simply trying to make a Roger Corman picture.5 Critical writing on Jaws has tended to exclude the Western from the film’s generic DNA. -

Melia-Ebrochure.Pdf
SOHNA - THE BEST OF SUBURBIA AND THE CITY Sohna or South Gurgaon is an Idyllic retreat with sulphur water Springs , Scenic Lake and a charming bird scantury, just a stone throw away. South of Gurgaon offers you the luxury of living in a chaos-free environment – while enjoying Gurgaon's best amenities at an affordable price compared to Gurgaon. South of Gurgaon is well connected to Gurgaon and the National Capital by the National Highway 248A which will also soon be revamped to a 6 Lane highway. The areas around the Gurgaon Sohna highway is set to emerge as the next axis of industrial and commercial development like Manesar. The Haryana State Industrial & Infrastructure Development Corporation (HSIIDC) has acquired about 1,700 acres of land in Roz Ka Meo, nearly 26 km from Gurgaon, to establish a new industrial township on the lines of IMT Manesar. It will be well connected by the in-progress KMP (Kundli-Manesar-Palwal) and DMIC (Delhi Mumbai Industrial Corridor). Coming to localized connectivity, Sohna has a proposed 60 meter wide sector road that connects 5 sectors of Sohna. It will also have a 2 km elevated road between Vatika City and Badshahpur. South of Gurgaon, is also rapidly accessible from Udyog Vihar, Cyber City, IFFCO Chowk, Rajeev Chowk, NH8, Subhash Chowk and Hero Honda Chowk. Infrastructure South of Gurgaon has many other important facilities already in place – like banks, ATMs, shops for daily needs, nearby – all of which make living here extremely convenient. Hospitals like Medanta, Artemis, Paras, Fortis, Max, etc. are also located within 25-30 minutes. -

PLANETARIAN Journal of the International Planetarium Society Vol
PLANETARIAN Journal of the International Planetarium Society Vol. 29, No.4, December 2000 Articles 6 Invitations for IPS 2004 ....................................................... various 12 Creation of A New World of 1.7 Million Stars .. Takayuki Ohira 16 We Make the Magic ........................................................ Jack Dunn 18 Planetarium Partnerships ....................................... Carole Helper Features 21 Reviews ...................................................................... April S. Whitt 26 Forum: How Can IPS Serve You in Future? .............. Steve 29 Mobile News Network ............................................. Susan 34 What's New ................................................................ Jim Manning 38 International News ..................................................... Lars Broman 43 President's Message .................................. oo .................. Dale Smith 61 Minutes of IPS Council Meeting ...................... Lee Ann Hennig 70 Jane's Corner ............................................................. Jane Hastings North America Welcomes a Brilliant NelN Character in Star ShOlNs: Zeiss Fiber Optics With the dawn of the new millenni improve the quality of Star Shows for um, visitors of the new planetariums in audiences of the Universarium. They are Oakland, CA and New York City will also offered with the Starmaster, the experience brilliant stars produced by medium planetarium. the Carl Zeiss Universarium fiber optics Quality at the highest level which systems, -

Melian Nymphs Scholars Widely Assume That Hesiod Alludes to An
Melian Nymphs Scholars widely assume that Hesiod alludes to an older tradition that human beings sprang from ash trees or their nymphs (West 1978, on WD 145-6, Most 1997,109- 110; Hardie 2000, 20; Yates 2004; Sistakou 2009, 253 n. 35): at Theog. 187, where the Meliae, like the Giants, are born from Gaia and Ouranos' blood, perhaps at Theog. 563, and at WD 145, where Zeus makes the Bronze Race from ash-trees. However, there really is no evidence for such a tradition before Hesiod; it is an interpretation of Hesiod's text. Hesiod narrates how Zeus withdrew fire at Theog. 563-4,: οὐκ ἐδίδου μελίῃσι πυρὸς μένος ἀκαμάτοιο θνητοῖς ἀνθρώποις οἳ ἐπὶ χθονὶ ναιετάουσιν He was not giving the power of tireless fire to the ash-trees for the benefit of mortal men who live on the earth Ancient commentators took μελίῃσι as a synonym for ἀνθρώποις, as the scholia show: 563b.) μ ε λ ί ῃ σ ι ν : ἀντὶ τοῦ μελιηγενὴς ἢ ἀπό τινος Μελίας οὕτω καλουμένης νύμφης. ἄλλως. ἢ ὅτι ἐξ ἀρχῆς ἐκ Μελιῶν νυμφῶν ἐγεννῶντο οἱ ἄνθρωποι ἢ ὅτι γεννώμενοι ἐρρίπτοντο ὑπὸ ταῖς μελίαις, ἤτοι τοῖς δένδροις. Ash-trees: instead of "ash-born" or from a certain nymph who was named this way, Melia. Or because in the beginning people were born from the Melian numphs or because at birth they were cast under ash- trees, or simply trees. We cannot know why ancient scholars preferred this interpretation instead of the simpler one that Zeus transmitted fire into trees (ash is an excellent firewood). -

Sleepcoacher: Combining Computational and Clinician-Generated Sleep Recommendations
SleepCoacher: Combining Computational and Clinician-Generated Sleep Recommendations An honors thesis submitted for the Bachelor of the Arts Degree in the Department of Computer Science at Brown University by Danae¨ Metaxa-Kakavouli May 2015 SLEEPCOACHER: COMBINING COMPUTATIONAL AND CLINICIAN-GENERATED SLEEP RECOMMENDATIONS Danae¨ Metaxa-Kakavouli Brown University 2015 Widespread use of smartphones to track various important aspects of personal health, including sleep, has the potential to empower individuals to better un- derstand and improve their health independently and easily. Access to raw data and visualizations, however, leaves much to be desired for these users. Simple, personalized recommendations incorporating input of healthcare pro- fessionals can improve the efficacy of self-tracking and help guide non-expert self-trackers. We present SleepCoacher, a system integrating personalized data- driven recommendations with feedback from professional sleep clinicians. To evaluate SleepCoacher, we collected one month of smartphone-recorded sleep data from 24 undergraduate students. We collect one month of data for each participant, and in the last two weeks, we provide a personalized recommen- dations to each user every three days. We use the data collected to measure the effect of recommendations on various aspects of sleep including total hours of sleep, daily self-reported sleep quality, frequency of overnight awakenings, and sleep onset latency. We find that following recommendations significantly im- proves aspects of sleep hygiene in 94% of users, improved the specific aspects of sleep being targeted by these recommendations in 61% of users. This combina- tion of clinical input and user data is the first of its kind, and in the future can be combined into a fully automated system that provides effective and actionable individualized recommendations in real time based on observed improvements in other users. -

Melia Azedarach L
Journal of Bacteriology & Mycology: Open Access Research Article Open Access Formulation of Allium sativum L. and Melia azedarach L. plant extracts and their effects on Myzus persicae Sulzer, 1776 (Hemiptera: Aphididae) Abstract Volume 8 Issue 3 - 2020 Myzus persicae Sulzer, 1776 (Hem.: Aphididae) the most important pests of vegetable Pervin ERDOGAN,1 Pelin AKSU,2 Gamze cultivated in the world. Pesticides are used extensively to control this pest. Intensive use ESİN KILINC,2 Murat KAHYAOGLU,3 of chemical pesticides to control pests caused various side effects such as residues in the 2 product, pests’ resistance and push a great risk for human health, nature and environment. Numan E BABAROGLU 1 This study was undertaken to provide an alternative to chemical pesticides. For this purpose, Department of Plant Protection, Sivas Science and Technology University, Turkey the extract of Allium sativum L. (Liliaceae) and Melia azedarach L. (Meliaceae) were 2Plant Protection Central Research Institute, Turkey prepared. Then formulation studies of these extracts were carried out with several inert 3Agriculture and Chemical industry Trade Corporation, Turkey ingredients. Obtained preparations were subjected to quality control tests in the laboratory. As a result of these tests, preparations which were found successfully were separated/ Correspondence: Pervin ERDOGAN, Department of Plant chosen for effectiveness studies on M. persicae. According to the results of laboratory Protection, Faculty of Agricultural Sciences and Technology, Sivas studies, the highest dose found to be effective and theirs two upper doses (10, 15, 20ml/L) Science and Technology University, Turkey, Tel +90346 219 13 98, were taken to examine effect on M. persicae at the greenhouse conditions. -

Robert Graves the White Goddess
ROBERT GRAVES THE WHITE GODDESS IN DEDICATION All saints revile her, and all sober men Ruled by the God Apollo's golden mean— In scorn of which I sailed to find her In distant regions likeliest to hold her Whom I desired above all things to know, Sister of the mirage and echo. It was a virtue not to stay, To go my headstrong and heroic way Seeking her out at the volcano's head, Among pack ice, or where the track had faded Beyond the cavern of the seven sleepers: Whose broad high brow was white as any leper's, Whose eyes were blue, with rowan-berry lips, With hair curled honey-coloured to white hips. Green sap of Spring in the young wood a-stir Will celebrate the Mountain Mother, And every song-bird shout awhile for her; But I am gifted, even in November Rawest of seasons, with so huge a sense Of her nakedly worn magnificence I forget cruelty and past betrayal, Careless of where the next bright bolt may fall. FOREWORD am grateful to Philip and Sally Graves, Christopher Hawkes, John Knittel, Valentin Iremonger, Max Mallowan, E. M. Parr, Joshua IPodro, Lynette Roberts, Martin Seymour-Smith, John Heath-Stubbs and numerous correspondents, who have supplied me with source- material for this book: and to Kenneth Gay who has helped me to arrange it. Yet since the first edition appeared in 1946, no expert in ancient Irish or Welsh has offered me the least help in refining my argument, or pointed out any of the errors which are bound to have crept into the text, or even acknowledged my letters. -

Defining Orphism: the Beliefs, the Teletae and the Writings
Defining Orphism: the Beliefs, the teletae and the Writings Anthi Chrysanthou Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds School of Languages, Cultures and Societies Department of Classics May 2017 The candidate confirms that the work submitted is his/her own and that appropriate credit has been given where reference has been made to the work of others. I This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. © 2017 The University of Leeds and Anthi Chrysanthou. The right of Anthi Chrysanthou to be identified as Author of this work has been asserted by her in accordance with the Copyright, Designs and Patents Act 1988. II Acknowledgements This research would not have been possible without the help and support of my supervisors, family and friends. Firstly, I would like to express my sincere gratitude to my supervisors Prof. Malcolm Heath and Dr. Emma Stafford for their constant support during my research, for motivating me and for their patience in reading my drafts numerous times. It is due to their insightful comments and constructive feedback that I have managed to evolve as a researcher and a person. Our meetings were always delightful and thought provoking. I could not have imagined having better mentors for my Ph.D studies. Special thanks goes to Prof. Malcolm Heath for his help and advice on the reconstruction of the Orphic Rhapsodies. I would also like to thank the University of Leeds for giving me the opportunity to undertake this research and all the departmental and library staff for their support and guidance. -

(1756- 1791) Apollo Et Hyacinthus, K.38
WOLFGANG AMADEUS MOZART (1756-1791) APOLLO ET HYACINTHUS, K.38 Latin intermedium in three parts (1767) Libretto by RUFINUS WIDL (1731-1798) OEBALUS King of Laconia ANDREW KENNEDY tenor MELIA daughter of Oebalus KLARA EK soprano HYACINTHUS son of Oebalus SOPHIE BEVAN soprano APOLLO guest of Oebalus LAWRENCE ZAZZO countertenor ZEPHYRUS friend of Hyacinthus CHRISTOPHER AINSLIE countertenor TWO PRIESTS OF APOLLO MARCUS FARNSWORTH baritone DAVID SHIPLEY bass THE MOZARTISTS Leader: Matthew Truscott Continuo: Steven Devine (harpsichord), Joseph Crouch (cello), Cecelia Bruggemeyer (double bass) IAN PAGE conductor MOZART / APOLLO ET HYACINTHUS 3 8536_CLA_Apollo_Signum_BOOKLET_134x122_TEXT PAGES_FINAL.indd 3 16/04/2019 15:26 4 MOZART / APOLLO ET HYACINTHUS 8536_CLA_Apollo_Signum_BOOKLET_134x122_TEXT PAGES_FINAL.indd 4 16/04/2019 15:26 APOLLO ET HYACINTHUS, K.38 PROLOGUS Page 1 Intrada 2’41 23 2 Recitativo: Amice! iam parata sunt omnia (Hyacinthus, Zephyrus, Oebalus, Melia) 2’53 23 3 No. 1, Chorus: Numen o Latonium (Chorus, Oebalus) 4’26 25 4 Recitativo: Heu me! periimus! (Melia, Oebalus, Hyacinthus, Zephyrus) 1’24 26 5 No. 2, Aria: Saepe terrent Numina (Hyacinthus) 7’48 27 6 Recitativo: Ah Nate! vera loqueris (Oebalus, Apollo, Hyacinthus, Melia, Zephyrus) 3’04 27 7 No. 3, Aria: Iam pastor Apollo (Apollo) 3’46 30 CHORUS I 8 Recitativo: Amare numquid Filia (Oebalus, Melia) 1’49 31 9 No. 4, Aria: Laetari, iocari (Melia) 6’39 32 10 Recitativo: Rex! de salute Filii (Zephyrus, Oebalus, Melia) 5’22 33 11 No. 5, Aria: En! duos conspicis (Zephyrus) 3’08 37 12 Recitativo: Heu! Numen! ecce! (Zephyrus, Melia, Apollo) 2’05 37 13 No. 6, Duetto: Discede crudelis! (Melia, Apollo) 6’22 39 CHORUS II 14 Recitativo accompagnato: Non est – Quis ergo Nate! (Hyacinthus, Oebalus) 2’35 40 15 No. -
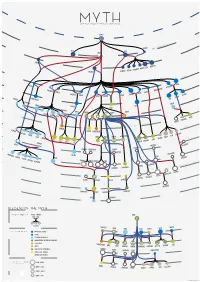
Legend of the Myth
MYTH Graph of greek mythological figures CHAOS s tie dei ial ord EREBUS rim p EROS NYX GAEA PONTU S ESIS NEM S CER S MORO THANATO A AETHER HEMER URANUS NS TA TI EA TH ON PERI IAPE HY TUS YNE EMNOS MN OC EMIS EANUS US TH TETHYS COE LENE PHOEBE SE EURYBIA DIONE IOS CREUS RHEA CRONOS HEL S EO PR NER OMETH EUS EUS ERSE THA P UMAS LETO DORIS PAL METIS LAS ERIS ST YX INACHU S S HADE MELIA POSEIDON ZEUS HESTIA HERA P SE LEIONE CE A G ELECTR CIR OD A STYX AE S & N SIPH ym p PA hs DEMETER RSES PE NS MP IA OLY WELVE S EON - T USE OD EKATH M D CHAR ON ARION CLYM PERSEPHONE IOPE NE CALL GALA CLIO TEA RODITE ALIA PROTO RES ATHENA APH IA TH AG HEBE A URAN AVE A DIKE MPHITRIT TEMIS E IRIS OLLO AR BIA AP NIKE US A IO S OEAGR ETHRA THESTIU ATLA S IUS ACRIS NE AGENOR TELEPHASSA CYRE REUS TYNDA T DA AM RITON LE PHEUS BROS OR IA E UDORA MAIA PH S YTO PHOEN OD ERYTHE IX EUROPA ROMULUS REMUS MIG A HESP D E ERIA EMENE NS & ALC UMA DANAE H HARMONIA DIOMEDES ASTOR US C MENELA N HELE ACLES US HER PERSE EMELE DRYOPE HERMES MINOS S E ERMION H DIONYSUS SYMAETHIS PAN ARIADNE ACIS LATRAMYS LEGEND OF THE MYTH ZEUS FAMILY IN THE MYTH FATHER MOTHER Zeus IS THEM CHILDREN DEMETER LETO HERA MAIA DIONE SEMELE COLORS IN THE MYTH PRIMORDIAL DEITIES TITANS SEA GODS AND NYMPHS DODEKATHEON, THE TWELVE OLYMPIANS DIKE OTHER GODS PERSEPHONE LO ARTEMIS HEBE ARES HERMES ATHENA APHRODITE DIONYSUS APOL MUSES EUROPA OSYNE GODS OF THE UNDERWORLD DANAE ALCEMENE LEDA MNEM ANIMALS AND HYBRIDS HUMANS AND DEMIGODS circles in the myth 196 M - 8690 K } {Google results M CALLIOPE INOS PERSEU THALIA CLIO 8690 K - 2740 K S HERACLES HELEN URANIA 2740 K - 1080 K 1080 K - 2410 J.