A Model for Structuring Biotechs and Developing Better Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

9894 Pharma Tech Media Planner V6 2007
www.pharmtech.com years 1977– 2007 30ANNIVERSARY CELEBRATING 30 YEARS AS THE 2007 INDUSTRY’S MOST AUTHORITATIVE SOURCE Media Planner years 1977–2007 ANNIVERSARY years 1977–2007 ANNIVERSARY THE PHARMACEUTICAL TECHNOLOGY BRAND PUBLISHER’S STATEMENT Pharmaceutical Technology’s authoritative reputation and powerful brand recognition within the pharmaceutical/biopharmaceutical development & manufacturing marketplace will help you establish and maintain your own strong brand among pharma industry decision makers. A circulation of 38,667 BPA-qualified subscribers* and unmatched peer written and reviewed editorial make Pharmaceutical Technology an invaluable resource within top pharma companies, as well as small, specialty and biotech pharma companies spending billions each year on pharmaceutical development and manufacturing. Please celebrate with us as Pharmaceutical Technology marks its 30th Anniversary as the industry leader. —Michael Tracey, Publisher % 90 of readers rated Pharmaceutical Technology as important or very important to them as a professionalˆ EDITORIAL MISSION Pharmaceutical Technology publishes authoritative, reliable, and timely peer-reviewed research and expert analyses for scientists, engineers, technicians, and managers engaged in process development, manufacturing, formulation, analytical technology, packaging and regulatory compliance in the pharmaceutical and biotechnology industries. —Douglas McCormick, Editor in chief www.pharmtech.com *BPA June 2006 Statement ^2006 Readership Study Conducted by Advanstar Research -

Memorandum JUL 1 6 201D
Memorandum Subject Date Additional Quota Letters Received by July 15, 2010 (DFN: 630-08.2) JUL 1 6 201D To From Christine A. Sanncrud. Ph.D., Chief "Barbar• J.Illoockholdt, Chief Drug & Chemical Evaluation Section Regul• torn Section Office of Diverison Control Off cL rl Diversion Control On July 15. 2010, this section received your e-mail requesting a review of seventeen (17) quota applications from sixteen (16) registered manufacturers to determine if there arc any pending administrative/legal actions against these applicants and to advise ODE of the findings. ODOR conducted reviews (NADDIS, CSA, etc), as well as surveyed the responsible field offices for their input and recommendations. Provided below are the results and recommendations. QUOTA APPLICANTS wan NO ADVERSE OR DEROGATORY INFORMATIO N Novartis Consumer I lealth Lincoln (10345) (b)(4);(b)(7)(E) Baxter (10346) Generics Bidco li bda Vintage (10347) Noramco Delaware (10348) Pharmaceuticals International inc. (10349) Pharmedium (10351) Pharmedium (10352) Rhodes (10356) Bio-Pharm (10357) Patheon (10358) Patheon (10359) Watson (10361) B & B (10363) lospira. Inc. NC (10364) Epic Pharma (10366) Mallinckrodt I lobart (10367) Chemtos (10368) Vol. II Page 55 2 Per consultation with the field offices. DEA does not have sufficient grounds to limit, restrict. or deny quota requests from these registrants. Based on this information. ODG suggests that you proceed with the completion of the quota applications. If you have any questions pertaining to this information. please feel free to contact me (b)(6);(b)(7)( or SC C) (b)(6);(b)(7)(C) Vol. II Page 56 Memorandum Subject Date Additional Quota Letters Received as of July 19, 201() (DFN: 630-08.2) stir 2 8 2010,., To Fr ,./"./ Christine A. -

Pharmaceutical Research and Manufacturers of America – Phrma
The Short-Term and Long-Term Competitive Impact of Authorized Generics A Report for the Federal Trade Commission October 28, 2009 TABLE OF CONTENTS INTRODUCTION ........................................................................................................................... 1 DISCUSSION .................................................................................................................................. 3 I. LONG-TERM COMPETITIVE HARM IS UNLIKELY ................................................... 3 A. Unsupported Foreclosure Claims Have Been Made For Nearly Two Decades .................................................................................................................... 4 B. Marketplace Realities Undercut The Long-Tenn Foreclosure Theory .................... 7 1. Sales and Profitability Are Growing ........................................................... 7 2. Wall Street Valuations Are Growing ........................................................ 10 II. THE FTC PRICING ANALYSIS SHOWS $880 MILLION IN CONSUMER SAVINGS .......................................................................................................................... 14 III. CONSUMER SAVINGS SHOULD BE MEASURED USING WHOLESALE DATA AND WEIGHTED AVERAGE PRICES .............................................................. 17 A. The Wholesale Data Carries Far More Weight.. .................................................... 17 B. Average Drug Prices Should Be Volume-Weighted ............................................. 19 IV. -

Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Paten
Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patents Drugs Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Patent DrugsDrug Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Under Patent Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under P Drugs Under Patent Drugs Under Patent Drugs Under Patent Drugs Under -

Clinical Research Services
Clinical Research Services Advancing the future of medicine through research. Who We Are Experience Clinical Research Services is a department within Clinical Research Services has been involved CHI St. Alexius Health in Bismarck, ND. We are fully in clinical research since 1987. Our professional dedicated to providing research support services staff has more than 180 years of combined to physicians within our region and the valued experience in clinical trials. We are committed patients they serve. to providing outstanding service to ensure the success of every project. CHI St. Alexius Health is a tertiary care facility affiliated with PrimeCare, a health group which Our involvement in inpatient and outpatient combines the most experienced, trusted, and phase II-IV clinical and device research studies has proven medical leaders in the area. PrimeCare is contributed substantially to the approval of new comprised of a network which includes more than drugs and treatments. You should carefully consider 190 physicians in private and institutional practice, both the benefits and the risks of participation including: CHI St. Alexius Health, Mid Dakota Clinic, before enrolling in a study. The Bone & Joint Center, and other affiliated area physicians. Many of these physicians are actively Previous clinical study trials: involved as investigators for clinical trials. • Oncology • Orthopaedic • Rheumatology • Diabetes Our clinical research team is committed to providing: • Neurology • Weight loss • Rapid study start-up • Cardiology • Pain • Pro-active patient enrollment • Urology • Men’s Health • Clean data submission • Gastroenterology • Women’s Health • Highly experienced principal • Infectious Disease • Medical Devices investigators • Pediatric • Initial training and continuing education for all support personnel Sponsors Patient Demographics Abbott Laboratories Characteristics of our patients include Acorda Therapeutics, Inc. -

Dr. Richard Rozek
Dear Ms. Overstreet: I am interested in being considered for the vacancy on the Glynn Brunswick Memorial Hospital Authority. Per the instructions with the vacancy announcement, I attached a copy of my vita to the letter. I am an economist with a specialty in health care economics. I have worked on competition, regulation, contract, and tax issues in health care during my career in academic, federal government, and private sector positions. I have written articles on health care issues and testified in major health care litigations. I have owned a home on Jekyll Island since 1993. Please let me know if you need additional information. Sincerely, Richard P. Rozek, Ph.D. RICHARD P. ROZEK, PH.D. CONTACT INFORMATION Redacted Jekyll Island, GA 31527 Phone: 912 Redacted Redacted gmail.com BACKGROUND Dr. Rozek received a B.A. degree in Mathematics with honors from the College of St. Thomas, a M.A. degree in Mathematics from the University of Minnesota, and M.A. and Ph.D. degrees in Economics from the University of Iowa. Dr. Rozek began his professional career as an Assistant Professor at the University of Pittsburgh where he taught courses in industrial organization, mathematical economics, and microeconomic theory. Dr. Rozek then worked for over six years in the Bureau of Economics at the Federal Trade Commission in a series of senior staff positions including Deputy Assistant Director for Antitrust. While at the FTC, Dr. Rozek evaluated antitrust and regulatory issues in electric and gas utilities, oil pipelines, soft drinks, for-profit and nonprofit hospitals, motion pictures, pharmaceuticals, and information industries. -

Inside This Issue
Issue No. 225 FDA Counting on Global Strategy April 2011 To Better Secure Supply Chain The FDA’s work toward tighter supply chain control through INsIde thIs Issue international regulatory cooperation is falling short, but a new global strategy may speed up the agency’s progress, a top FDA Sterility issues lead to an- official says. other J&J recall ........Page 2 The next several years are critical in the agency’s transformation FDA zeroes in on compli- to a “global agency,” John Taylor, the FDA’s acting principal deputy ance history to make in- commissioner, said March 14 at a Pew Charitable Trusts conference spection decisions ....Page 3 in Washington, D.C., on drug supply safety. To achieve adequate supply chain control, Taylor said, the FDA 483 Insider ..............Page 5 needs novel and updated enforcement tools, a global alliance of reg- ulators, new authorities to create proactive tools for product safety, APP recalls oncologic irino- tecan over fungal contami- (See Global, Page 2) nation .......................Page 6 McNeil Gets Consent Decree After Pharma manufacturing in Period of Manufacturing Woes Japan largely spared by The FDA has said enough is enough to McNeil Consumer Health- quake ........................Page 8 care over its repeated manufacturing problems, issuing a consent decree that indefinitely closes one plant and places oversight over two others. Bill would increase penal- ties for prescription drug The agency said the Johnson & Johnson subsidiary, which has thefts ......................Page 10 been plagued with recalls of its OTC drugs relating to cGMP viola- tions, will not be able to reopen its troubled Fort Washington, Pa., Dakota labs gets warning plant until a litany of changes have been made. -

SECURITIES CLASS ACTIONS in the LIFE SCIENCES SECTOR 2015 Annual Survey SECURITIES CLASS ACTIONS in the LIFE SCIENCES SECTOR 2015 Annual Survey
SECURITIES CLASS ACTIONS IN THE LIFE SCIENCES SECTOR 2015 Annual Survey SECURITIES CLASS ACTIONS IN THE LIFE SCIENCES SECTOR 2015 Annual Survey INTRODUCTION AND OVERVIEW 1 Decisions Issued in 2015 –Trends and Analysis 3 TABLE AND SHORT SUMMARIES OF 2015 DECISIONS 13 DETAILED SUMMARIES OF 2015 DECISIONS DECISIONS RELATED TO DEVELOPMENT-STAGE DRUGS OR DEVICES 19 Appellate Decisions 20 District Court Decisions: Motion to Dismiss or for Summary Judgment Granted 22 District Court Decisions: Motion to Dismiss or for Summary Judgment Denied 29 Decisions Related to Stock Promotion Activities 32 DECISIONS RELATED TO POST-APPROVAL DRUGS OR DEVICES Launch Issues 36 Regulatory Issues Appellate Decisions 36 District Court Decisions: Motion to Dismiss or for Summary Judgment Granted 37 District Court Decisions: Motion to Dismiss or for Summary Judgment Denied 39 Non-Regulatory Issues 41 TABLE OF NEW FILINGS IN 2015 43 ABOUT THE PRACTICE 57 Sidley Securities Class Actions in the Life Sciences Sector | 2015 Annual Survey INTRODUCTION AND OVERVIEW This year-in-review addresses developments in securities class actions brought against life sciences companies in 2015. We begin with an overview and analysis of trends in decisions involving life sciences companies with products at two distinct stages of development—pre- and post-FDA approval. Next, we provide summaries of the 34 federal district court and appellate court decisions surveyed. Finally, we catalog the new securities class action complaints filed against life sciences companies in 2015. At the most basic level the cases analyzed share a common feature. In each, a life sciences company suffered a setback that, when publicized, was followed first by a stock price decline and then by litigation initiated by shareholders seeking to recover investment losses. -
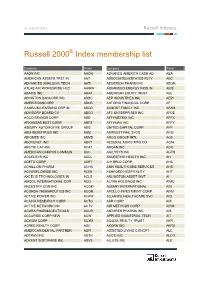
Russell 2000 Growth Index Membership List
As of 06/27/2011 Russell Indexes. Russell 2000® Index membership list Company Ticker Company Ticker AAON INC AAON ADVANCE AMERICA CASH AD AEA AMERICAN ASSETS TRST IN AAT ASSOCIATED ESTATES RLTY AEC ADVANCED ANALOGIC TECH AATI AEGERION PHARM INC AEGR ATLAS AIR WORLDWIDE HLD AAWW ADVANCED ENERGY INDS IN AEIS ABAXIS INC ABAX AMERICAN EQUITY INVST AEL ABINGTON BANCORP INC ABBC AEP INDUSTRIES INC AEPI AMERIS BANCORP ABCB ASTORIA FINANCIAL CORP AF CAMBIUM LEARNING GRP IN ABCD ALMOST FAMILY INC AFAM ADVISORY BOARD CO ABCO AFC ENTERPRISES INC AFCE ACCO BRANDS CORP ABD AFFYMETRIX INC AFFX ARKANSAS BEST CORP ABFS AFFYMAX INC AFFY ASBURY AUTOMOTIVE GROUP ABG UNITED CAPITAL CORP AFP ABM INDUSTRIES INC ABM AMTRUST FINL SVCS AFSI ABIOMED INC ABMD ARGO GROUP INTL AGII ABOVENET INC ABVT FEDERAL AGRIC MTG CO AGM ARCTIC CAT INC ACAT ARGAN INC AGX AMERICAN CAMPUS COMMUN ACC AGILYSYS INC AGYS ACCELRYS INC ACCL ACCRETIVE HEALTH INC AH ACETO CORP ACET A H BELO CORP AHC ACHILLION PHARM ACHN AMN HEALTHCARE SERVICES AHS ACI WORLDWIDE INC ACIW ASHFORD HOSPITALITY AHT AXCELIS TECHNOLOGIES IN ACLS ARLINGTON ASSET INVT AI AMCOL INTERNATIONAL COR ACO ALTRA HOLDINGS INC AIMC ANCESTRY.COM INC ACOM ALBANY INTERNATIONAL AIN ACORDA THERAPEUTICS INC ACOR APOLLO INVESTMENT CORP AINV ACTIVE POWER INC ACPW ALLIANCE HEALTHCARE SVC AIQ ACACIA RESEARCH CORP ACTG AAR CORP AIR ACTIVE NETWORK INC ACTV AIR METHODS CORP AIRM ACURA PHARMACEUTICALS ACUR ANTARES PHARMA INC AIS ACCURIDE CORP NEW ACW APPLIED INDUSTRIAL TECH AIT ACXIOM CORP ACXM ACADIA REALTY TRUST AKR AGREE REALTY CORP ADC AKORN INC AKRX AMERICAN DENTAL PARTNER ADPI ASSISTED LIVING CONCPT ALC ADTRAN INC ADTN ALICO INC ALCO ADVENT SOFTWARE INC ADVS ALLETE INC ALE As of 06/27/2011 Russell Indexes. -

Twenty-Seven Years of Pharmaceutical Industry Criminal and Civil Penalties: 1991 Through 2017
Twenty-Seven Years of Pharmaceutical Industry Criminal and Civil Penalties: 1991 Through 2017 March 14, 2018 Sammy Almashat, M.D., M.P.H. Ryan Lang, M.D., M.P.H. Sidney M. Wolfe, M.D. Michael Carome, M.D. ––––––––––––––––––– www.citizen.org Public Citizen Pharmaceutical Industry Settlements: 1991-2017 About Public Citizen Public Citizen is a national nonprofit organization with more than 400,000 members and supporters. We represent consumer interests through lobbying, litigation, administrative advocacy, research, and public education on a broad range of issues, including consumer rights in the marketplace, product safety, financial regulation, safe and affordable health care, campaign finance reform and government ethics, fair trade, climate change, and corporate and government accountability. 2 Public Citizen Pharmaceutical Industry Settlements: 1991-2017 Table of Contents Executive Summary 4 Introduction 7 Methods 7 Results 7 Combined federal and state trends 7 Federal settlements 8 State settlements 8 Civil versus criminal settlements 10 False Claims Act and qui tam settlements 10 Worst and repeat offenders and largest settlements 11 Types of violations 11 Discussion 12 Federal settlements 13 State settlements 17 More enforcement needed 19 Limitations and future research 23 Conclusion 23 Appendix 1: Figures and Tables 25 Appendix 2: Detailed methodology 52 3 Public Citizen Pharmaceutical Industry Settlements: 1991-2017 Executive Summary Background Public Citizen has published three previous reports — in 2010,1 2012,2 and 20163 — documenting the number and size of criminal and civil settlements and court judgments reached between the federal and state4 governments and pharmaceutical manufacturers. The 2016 report, which included all settlements from 1991 through 2015, revealed that the pace of settlement activity had decreased considerably in the then-most-recent two-year period. -

Russia, Eastern Europe Growing on the Generics Scene
April 17, 2008 Russia, Eastern Europe growing on the generics scene Amy Brown While dominance of the generics scene in terms of scale is set to remain in the hands of Teva, Novartis’s Sandoz and Mylan, and Indian firms have been making inroads for some time, companies emerging from Eastern Europe and Russia are increasingly moving up the rankings and featuring at the top of sales growth tables. EvaluatePharma’s Peer Group Analyzer shows that in terms of unbranded prescription sales growth over the next five years, Russian firms in particular are doing well at the top of the table, as spending on healthcare in the region grows (see table below). IMS recently reported that Russia was the fastest growing pharmaceutical market in the European region in 2007, expanding by 20%. Market WW Unbranded Generic Sales - ranked on growth rate Rank Compound Annual Sales in 2012 # Company Location Growth Rate 2007 2012 (US$m) (2007 to 2012) 1 Bioton Poland 51% 43 36 203 2 Veropharm Russia 32% 36 27 453 3 IMPAX Laboratories USA 24% 35 29 381 4 Mylan USA 21% 4 3 4,922 5 Pharmstandard Russia 21% 23 18 987 Glenmark 6 India 17% 25 19 823 Pharmaceuticals Czech 7 Zentiva 17% 18 15 1,354 Republic Sun Pharmaceutical 8 India 16% 19 16 1,243 Industries 9 Aurobindo Pharma India 15% 31 30 332 10 Matrix Laboratories India 15% 34 34 263 Top of the list is Poland’s Bioton, a small company which derives the majority of its sales from insulin and antibiotics. -

Scott-Macon Healthcare Review: Third Quarter 2011
SCOTT-MACON HEALTHCARE REVIEW: THIRD QUARTER 2011 Third Quarter 2011 Healthcare Review 2 Healthcare Overview, Mean Revenue Multiples 4 Healthcare Overview, Mean EBITDA Multiples 5 Analysis of Selected Healthcare 6 Merger and Acquisition Transactions July 1 — September 30, 2011 Analysis of Selected 19 C O N T E N T S Publicly-Traded Healthcare Companies SCOTT-MACON, LTD. 2 THIRD QUARTER 2011 HEALTHCARE REVIEW Dear Clients and Friends, Scott-Macon is pleased to present our quar- Health for $600 million, making inVentiv one of the terly Healthcare Review covering the third quarter top contract research organizations in the world. of 2011. iSOFT Group has agreed to be acquired by CSC Pages four and five represent a visual snap- Computer Sciences in a transaction valued at $474 shot of the average multiples for all of the health- million, bolstering CSC’s healthcare information care, medical and pharmaceutical transactions that technology business. Interestingly, in the Insurance either closed or were announced during the third segment, every transaction in the quarter (in addition quarter, along with the average multiples for the ma- to October’s CIGNA/HealthSpring announced deal) jor publicly-traded healthcare, medical and pharma- involved government focused plans, as the market ceutical companies. continues to shy away from commercial insurers in the wake of healthcare reform legislation. Aetna Starting on page 6, we present information on continues diversifying away from its core insurance the quarter’s mergers and acquisitions activity. The business with its acquisition of PayFlex, a leader in largest deal in the Services segment this quarter and, the flexible spending area.