Body Mass As a Supertrait Linked to Abundance and Behavioral Dominance in Hummingbirds: a Phylogenetic Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Giant Hummingbird from Paramo De Chingaza, Colombia.-On 10 October 1981, During an Ornithological Survey at 3250 M Elev
GENERAL NOTES 661 E. nigrivestis is timed to coincide with flowering of P. huigrensis (and other plants) which bloom seasonally at lower elevations. The general ecology of E. nigrivestis is similar to that recorded for the closely related species, the Glowing Puffleg (E. vextitus)(Snow and Snow 1980). Snow and Snow (1980) observed E. vestitus feeding principally at plants of similar general morphology (straight tubular corollas 10-20 mm in length) in similar taxonomic groups (Cnvenclishia; Ericaceae more often than Palicourea; Rubiaceae for E. vestitus). Territoriality in E. vestitus was pronounced while this was only suggested in the male E. nigrivestis we observed, perhaps a seasonal effect. Finally E. vestitus, like E. nigrivestis, did not occur in tall forested habitats at similar elevations (Snow and Snow [1980] study at 2400-2500 m). The evidence presented here suggests that the greatly restricted range of E. nigrivestis is not due to dietary specializations. Male nectar sources are from a broad spectrum of plants, and the principal food plant of both sexes, P. huigrensis, is a widespread Andean species. Nesting requirements of E. nigrivestis may still be threatened with extinction through habitat destruction, especially if the species requires specific habitats of natural vegetation at certain times of year, such as that found on nude ridge crests. The vegetation on nudes in particular is rapidly disappearing because nudes provide flat ground for cultivation in otherwise pre- cipitous terrain. In light of its restricted range and the threat of habitat destruction resulting from such close proximity to a major urban center, Quito, we consider E. -

The Basilinna Genus (Aves: Trochilidae): an Evaluation Based on Molecular Evidence and Implications for the Genus Hylocharis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Revista Mexicana de Biodiversidad 85: 797-807, 2014 DOI: 10.7550/rmb.35769 The Basilinna genus (Aves: Trochilidae): an evaluation based on molecular evidence and implications for the genus Hylocharis El género Basilinna (Aves: Trochilidae): una evaluación basada en evidencia molecular e implicaciones para el género Hylocharis Blanca Estela Hernández-Baños1 , Luz Estela Zamudio-Beltrán1, Luis Enrique Eguiarte-Fruns2, John Klicka3 and Jaime García-Moreno4 1Museo de Zoología, Departamento de Biología Evolutiva, Facultad de Ciencias, Universidad Nacional Autónoma de México. Apartado postal 70- 399, 04510 México, D. F., Mexico. 2Departamento de Ecología Evolutiva, Instituto de Ecología, Universidad Nacional Autónoma de México. Apartado postal 70-275, 04510 México, D. F., Mexico. 3Burke Museum of Natural History and Culture, University of Washington, Box 353010, Seattle, WA, USA. 4Amphibian Survival Alliance, PO Box 20164, 1000 HD Amsterdam, The Netherlands. [email protected] Abstract. Hummingbirds are one of the most diverse families of birds and the phylogenetic relationships within the group have recently begun to be studied with molecular data. Most of these studies have focused on the higher level classification within the family, and now it is necessary to study the relationships between and within genera using a similar approach. Here, we investigated the taxonomic status of the genus Hylocharis, a member of the Emeralds complex, whose relationships with other genera are unclear; we also investigated the existence of the Basilinna genus. We obtained sequences of mitochondrial (ND2: 537 bp) and nuclear genes (AK-5 intron: 535 bp, and c-mos: 572 bp) for 6 of the 8 currently recognized species and outgroups. -
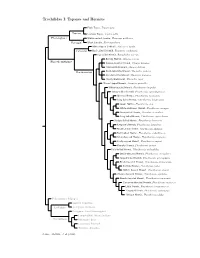
Topazes and Hermits
Trochilidae I: Topazes and Hermits Fiery Topaz, Topaza pyra Topazini Crimson Topaz, Topaza pella Florisuginae White-necked Jacobin, Florisuga mellivora Florisugini Black Jacobin, Florisuga fusca White-tipped Sicklebill, Eutoxeres aquila Eutoxerini Buff-tailed Sicklebill, Eutoxeres condamini Saw-billed Hermit, Ramphodon naevius Bronzy Hermit, Glaucis aeneus Phaethornithinae Rufous-breasted Hermit, Glaucis hirsutus ?Hook-billed Hermit, Glaucis dohrnii Threnetes ruckeri Phaethornithini Band-tailed Barbthroat, Pale-tailed Barbthroat, Threnetes leucurus ?Sooty Barbthroat, Threnetes niger ?Broad-tipped Hermit, Anopetia gounellei White-bearded Hermit, Phaethornis hispidus Tawny-bellied Hermit, Phaethornis syrmatophorus Mexican Hermit, Phaethornis mexicanus Long-billed Hermit, Phaethornis longirostris Green Hermit, Phaethornis guy White-whiskered Hermit, Phaethornis yaruqui Great-billed Hermit, Phaethornis malaris Long-tailed Hermit, Phaethornis superciliosus Straight-billed Hermit, Phaethornis bourcieri Koepcke’s Hermit, Phaethornis koepckeae Needle-billed Hermit, Phaethornis philippii Buff-bellied Hermit, Phaethornis subochraceus Scale-throated Hermit, Phaethornis eurynome Sooty-capped Hermit, Phaethornis augusti Planalto Hermit, Phaethornis pretrei Pale-bellied Hermit, Phaethornis anthophilus Stripe-throated Hermit, Phaethornis striigularis Gray-chinned Hermit, Phaethornis griseogularis Black-throated Hermit, Phaethornis atrimentalis Reddish Hermit, Phaethornis ruber ?White-browed Hermit, Phaethornis stuarti ?Dusky-throated Hermit, Phaethornis squalidus Streak-throated Hermit, Phaethornis rupurumii Cinnamon-throated Hermit, Phaethornis nattereri Little Hermit, Phaethornis longuemareus ?Tapajos Hermit, Phaethornis aethopygus ?Minute Hermit, Phaethornis idaliae Polytminae: Mangos Lesbiini: Coquettes Lesbiinae Coeligenini: Brilliants Patagonini: Giant Hummingbird Lampornithini: Mountain-Gems Tro chilinae Mellisugini: Bees Cynanthini: Emeralds Trochilini: Amazilias Source: McGuire et al. (2014).. -

1 AOS Classification Committee – North and Middle America Proposal Set 2020-A 4 September 2019 No. Page Title 01 02 Change Th
AOS Classification Committee – North and Middle America Proposal Set 2020-A 4 September 2019 No. Page Title 01 02 Change the English name of Olive Warbler Peucedramus taeniatus to Ocotero 02 05 Change the generic classification of the Trochilini (part 1) 03 11 Change the generic classification of the Trochilini (part 2) 04 18 Split Garnet-throated Hummingbird Lamprolaima rhami 05 22 Recognize Amazilia alfaroana as a species not of hybrid origin, thus moving it from Appendix 2 to the main list 06 26 Change the linear sequence of species in the genus Dendrortyx 07 28 Make two changes concerning Starnoenas cyanocephala: (a) assign it to the new monotypic subfamily Starnoenadinae, and (b) change the English name to Blue- headed Partridge-Dove 08 32 Recognize Mexican Duck Anas diazi as a species 09 36 Split Royal Tern Thalasseus maximus into two species 10 39 Recognize Great White Heron Ardea occidentalis as a species 11 41 Change the English name of Checker-throated Antwren Epinecrophylla fulviventris to Checker-throated Stipplethroat 12 42 Modify the linear sequence of species in the Phalacrocoracidae 13 49 Modify various linear sequences to reflect new phylogenetic data 1 2020-A-1 N&MA Classification Committee p. 532 Change the English name of Olive Warbler Peucedramus taeniatus to Ocotero Background: “Warbler” is perhaps the most widely used catch-all designation for passerines. Its use as a meaningful taxonomic indicator has been defunct for well over a century, as the “warblers” encompass hundreds of thin-billed, insectivorous passerines across more than a dozen families worldwide. This is not itself an issue, as many other passerine names (flycatcher, tanager, sparrow, etc.) share this common name “polyphyly”, and conventions or modifiers are widely used to designate and separate families that include multiple groups. -

Flowers Visited by Hummingbirds in an Urban Cerrado Fragment, Mato Grosso Do Sul, Brazil
Biota Neotrop., vol. 13, no. 4 Flowers visited by hummingbirds in an urban Cerrado fragment, Mato Grosso do Sul, Brazil Waldemar Guimarães Barbosa-Filho1,2 & Andréa Cardoso de Araujo1 1Laboratório de Ecologia, Centro de Ciências Biológicas e da Saúde, Universidade Federal de Mato Grosso do Sul – UFMS, CP 549, CEP 79070-900, Campo Grande, MS, Brasil. http://www-nt.ufms.br/ 2Corresponding author: Waldemar Guimarães Barbosa-Filho, e-mail: [email protected] BARBOSA-FILHO, W.G. & ARAUJO, A.C. Flowers visited by hummingbirds in an urban Cerrado fragment, Mato Grosso do Sul, Brazil. Biota Neotrop. 13(4): http://www.biotaneotropica.org.br/v13n4/en/ abstract?article+bn00213042013 Abstract: Hummingbirds are the main vertebrate pollinators in the Neotropics, but little is known about the interactions between hummingbirds and flowers in areas of Cerrado. This paper aims to describe the interactions between flowering plants (ornithophilous and non-ornithophilous species) and hummingbirds in an urban Cerrado remnant. For this purpose, we investigated which plant species are visited by hummingbirds, which hummingbird species occur in the area, their visiting frequency and behavior, their role as legitimate or illegitimate visitors, as well as the number of agonistic interactions among these visitors. Sampling was conducted throughout 18 months along a track located in an urban fragment of Cerrado vegetation in Campo Grande, Mato Grosso do Sul, Brasil. We found 15 species of plants visited by seven species of hummingbirds. The main habit for ornithophilous species was herbaceous, with the predominance of Bromeliaceae; among non-ornithophilous most species were trees from the families Vochysiaceae and Malvaceae. -
![Downloaded from Birdtree.Org [48] to Take Into Account Phylogenetic Uncertainty in the Comparative Analyses [67]](https://docslib.b-cdn.net/cover/5125/downloaded-from-birdtree-org-48-to-take-into-account-phylogenetic-uncertainty-in-the-comparative-analyses-67-245125.webp)
Downloaded from Birdtree.Org [48] to Take Into Account Phylogenetic Uncertainty in the Comparative Analyses [67]
bioRxiv preprint doi: https://doi.org/10.1101/586362; this version posted November 19, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. RESEARCH ARTICLE Open Access Distribution of iridescent colours in Open Peer-Review hummingbird communities results Open Data from the interplay between Open Code selection for camouflage and communication Cite as: preprint Posted: 15th November 2019 Hugo Gruson1, Marianne Elias2, Juan L. Parra3, Christine Recommender: Sébastien Lavergne Andraud4, Serge Berthier5, Claire Doutrelant1, & Doris Reviewers: Gomez1,5 XXX Correspondence: 1 [email protected] CEFE, Univ Montpellier, CNRS, Univ Paul Valéry Montpellier 3, EPHE, IRD, Montpellier, France 2 ISYEB, CNRS, MNHN, Sorbonne Université, EPHE, 45 rue Buffon CP50, Paris, France 3 Grupo de Ecología y Evolución de Vertrebados, Instituto de Biología, Universidad de Antioquia, Medellín, Colombia 4 CRC, MNHN, Ministère de la Culture et de la Communication, CNRS, Paris, France 5 INSP, Sorbonne Université, CNRS, Paris, France This article has been peer-reviewed and recommended by Peer Community In Evolutionary Biology Peer Community In Evolutionary Biology 1 of 33 bioRxiv preprint doi: https://doi.org/10.1101/586362; this version posted November 19, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Identification errors between closely related, co-occurring, species may lead to misdirected social interactions such as costly interbreeding or misdirected aggression. This selects for divergence in traits involved in species identification among co-occurring species, resulting from character displacement. -

Hummingbird (Family Trochilidae) Research: Welfare-Conscious Study Techniques for Live Hummingbirds and Processing of Hummingbird Specimens
Special Publications Museum of Texas Tech University Number xx76 19xx January XXXX 20212010 Hummingbird (Family Trochilidae) Research: Welfare-conscious Study Techniques for Live Hummingbirds and Processing of Hummingbird Specimens Lisa A. Tell, Jenny A. Hazlehurst, Ruta R. Bandivadekar, Jennifer C. Brown, Austin R. Spence, Donald R. Powers, Dalen W. Agnew, Leslie W. Woods, and Andrew Engilis, Jr. Dedications To Sandra Ogletree, who was an exceptional friend and colleague. Her love for family, friends, and birds inspired us all. May her smile and laughter leave a lasting impression of time spent with her and an indelible footprint in our hearts. To my parents, sister, husband, and children. Thank you for all of your love and unconditional support. To my friends and mentors, Drs. Mitchell Bush, Scott Citino, John Pascoe and Bill Lasley. Thank you for your endless encouragement and for always believing in me. ~ Lisa A. Tell Front cover: Photographic images illustrating various aspects of hummingbird research. Images provided courtesy of Don M. Preisler with the exception of the top right image (courtesy of Dr. Lynda Goff). SPECIAL PUBLICATIONS Museum of Texas Tech University Number 76 Hummingbird (Family Trochilidae) Research: Welfare- conscious Study Techniques for Live Hummingbirds and Processing of Hummingbird Specimens Lisa A. Tell, Jenny A. Hazlehurst, Ruta R. Bandivadekar, Jennifer C. Brown, Austin R. Spence, Donald R. Powers, Dalen W. Agnew, Leslie W. Woods, and Andrew Engilis, Jr. Layout and Design: Lisa Bradley Cover Design: Lisa A. Tell and Don M. Preisler Production Editor: Lisa Bradley Copyright 2021, Museum of Texas Tech University This publication is available free of charge in PDF format from the website of the Natural Sciences Research Laboratory, Museum of Texas Tech University (www.depts.ttu.edu/nsrl). -

'Bogotá' Type Specimens of the Hummingbird Genus Adelomyia
Thomas M. Donegan & Jorge Enrique Avendaño 195 Bull. B.O.C. 2015 135(3) ‘Bogotá’ type specimens of the hummingbird genus Adelomyia, with diagnosis of an overlooked subspecies from the East Andes of Colombia by Thomas M. Donegan & Jorge Enrique Avendaño Received 25 September 2014 Summary.—Molecular work has revealed that Speckled Hummingbirds Adelomyia melanogenys in the East Andes of dpto. Santander, Colombia, represent a distinct lineage not previously recognised taxonomically. Most specimens from this region differ from others taken in the East Andes by their more extensively rufous and speckled posterior underparts. Sound-recordings and biometrics showed broad overlap for all variables in both populations. Statistically significant but non- diagnosable differences exist in the number of notes in trills of songs, speed of calls and bill length. The type of Adelomyia melanogenys (Fraser 1840) is a ‘Bogotá’ specimen similar to birds from dpto. Cundinamarca, Colombia. Trochilus sabinae Bourcier & Mulsant, 1846, is also based on a ‘Colombia’ specimen. A possible type was identified that resembles the Santander population in its underparts.Adelomyia simplex Boucard, 1893, is based on a leucistic ‘Bogotá’ specimen more consistent with the Cundinamarca population than others. If the Santander population is recognised taxonomically, it is suggested to clarify the type locality for sabinae as the west slope of the East Andes in Santander or Boyacá, but molecular work is needed to confirm this. A. m. inornata in the southern Andes has a faster call and distinctive plumage, and perhaps merits species rank. The genus Adelomyia is monospecific, comprising the polytypic Speckled Hummingbird A. melanogenys, a widespread Andean hummingbird. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

The Best of Costa Rica March 19–31, 2019
THE BEST OF COSTA RICA MARCH 19–31, 2019 Buffy-crowned Wood-Partridge © David Ascanio LEADERS: DAVID ASCANIO & MAURICIO CHINCHILLA LIST COMPILED BY: DAVID ASCANIO VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM THE BEST OF COSTA RICA March 19–31, 2019 By David Ascanio Photo album: https://www.flickr.com/photos/davidascanio/albums/72157706650233041 It’s about 02:00 AM in San José, and we are listening to the widespread and ubiquitous Clay-colored Robin singing outside our hotel windows. Yet, it was still too early to experience the real explosion of bird song, which usually happens after dawn. Then, after 05:30 AM, the chorus started when a vocal Great Kiskadee broke the morning silence, followed by the scratchy notes of two Hoffmann´s Woodpeckers, a nesting pair of Inca Doves, the ascending and monotonous song of the Yellow-bellied Elaenia, and the cacophony of an (apparently!) engaged pair of Rufous-naped Wrens. This was indeed a warm welcome to magical Costa Rica! To complement the first morning of birding, two boreal migrants, Baltimore Orioles and a Tennessee Warbler, joined the bird feast just outside the hotel area. Broad-billed Motmot . Photo: D. Ascanio © Victor Emanuel Nature Tours 2 The Best of Costa Rica, 2019 After breakfast, we drove towards the volcanic ring of Costa Rica. Circling the slope of Poas volcano, we eventually reached the inspiring Bosque de Paz. With its hummingbird feeders and trails transecting a beautiful moss-covered forest, this lodge offered us the opportunity to see one of Costa Rica´s most difficult-to-see Grallaridae, the Scaled Antpitta. -

Diagnosis and Observations of a Hybrid Hummingbird (Metallura Tyrianthina X Aglaiocercus Kingi) in the Eastern Andes of Colombia F
Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 39(153):481-490, octubre-diciembre de 2015 Hybrid hummingbird at Rogitama, Boyacá doi: http://dx.doi.org/10.18257/raccefyn.260 Ciencias naturales Diagnosis and observations of a hybrid hummingbird (Metallura tyrianthina x Aglaiocercus kingi) in the Eastern Andes of Colombia F. Gary Stiles*, José Oswaldo Cortés-Herrera Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, Colombia Abstract An unusual, strikingly colored male hummingbird present for several months in the Rogitama Biodiversity Reserve in Boyacá, Colombia created considerable media attention. It was initially suggested that the bird might represent the rediscovery of the Bogotá Sunangel, Heliangelus zusii. However, a later study of its DNA identified H. zusii as a member of a clade that includes Aglaiocercus and Taphrolesbia. Given the many differences in plumage from the Rogitama hummingbird, this hypothesis was rejected. Three hypotheses regarding its identity were examined in detail: it could represent an unusually colored male of Aglaiocercus kingi, a hybrid between two local species, or an undescribed species. We observed this bird’s foraging and interactions with other hummingbirds, recorded its song and captured it to describe in detail its plumage and take morphological measurements, after which it was released. We rejected all hypotheses except that of a hybrid, and concluded that A. kingi and Metallura tyrianthina were its probable parents. In contrast to its plumage and morphometrics, its song was unlike those of its putative parents, possibly representing a transgressive character. The same may be true of the bluish coloration (termed “hybrid bluing”) that characterized several hybrids between green-backed parental species, including the Rogitama hummingbird. -

Programs and Field Trips
CONTENTS Welcome from Kathy Martin, NAOC-V Conference Chair ………………………….………………..…...…..………………..….…… 2 Conference Organizers & Committees …………………………………………………………………..…...…………..……………….. 3 - 6 NAOC-V General Information ……………………………………………………………………………………………….…..………….. 6 - 11 Registration & Information .. Council & Business Meetings ……………………………………….……………………..……….………………………………………………………………………………………………………………….…………………………………..…..……...….. 11 6 Workshops ……………………….………….……...………………………………………………………………………………..………..………... 12 Symposia ………………………………….……...……………………………………………………………………………………………………..... 13 Abstracts – Online login information …………………………..……...………….………………………………………….……..……... 13 Presentation Guidelines for Oral and Poster Presentations …...………...………………………………………...……….…... 14 Instructions for Session Chairs .. 15 Additional Social & Special Events…………… ……………………………..………………….………...………………………...…………………………………………………..…………………………………………………….……….……... 15 Student Travel Awards …………………………………………..………...……………….………………………………..…...………... 18 - 20 Postdoctoral Travel Awardees …………………………………..………...………………………………..……………………….………... 20 Student Presentation Award Information ……………………...………...……………………………………..……………………..... 20 Function Schedule …………………………………………………………………………………………..……………………..…………. 22 – 26 Sunday, 12 August Tuesday, 14 August .. .. .. 22 Wednesday, 15 August– ………………………………...…… ………………………………………… ……………..... Thursday, 16 August ……………………………………….…………..………………………………………………………………… …... 23 Friday, 17 August ………………………………………….…………...………………………………………………………………………..... 24 Saturday,