Free-Range Environment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Integrated Pest Management: Current and Future Strategies
Integrated Pest Management: Current and Future Strategies Council for Agricultural Science and Technology, Ames, Iowa, USA Printed in the United States of America Cover design by Lynn Ekblad, Different Angles, Ames, Iowa Graphics and layout by Richard Beachler, Instructional Technology Center, Iowa State University, Ames ISBN 1-887383-23-9 ISSN 0194-4088 06 05 04 03 4 3 2 1 Library of Congress Cataloging–in–Publication Data Integrated Pest Management: Current and Future Strategies. p. cm. -- (Task force report, ISSN 0194-4088 ; no. 140) Includes bibliographical references and index. ISBN 1-887383-23-9 (alk. paper) 1. Pests--Integrated control. I. Council for Agricultural Science and Technology. II. Series: Task force report (Council for Agricultural Science and Technology) ; no. 140. SB950.I4573 2003 632'.9--dc21 2003006389 Task Force Report No. 140 June 2003 Council for Agricultural Science and Technology Ames, Iowa, USA Task Force Members Kenneth R. Barker (Chair), Department of Plant Pathology, North Carolina State University, Raleigh Esther Day, American Farmland Trust, DeKalb, Illinois Timothy J. Gibb, Department of Entomology, Purdue University, West Lafayette, Indiana Maud A. Hinchee, ArborGen, Summerville, South Carolina Nancy C. Hinkle, Department of Entomology, University of Georgia, Athens Barry J. Jacobsen, Department of Plant Sciences and Plant Pathology, Montana State University, Bozeman James Knight, Department of Animal and Range Science, Montana State University, Bozeman Kenneth A. Langeland, Department of Agronomy, University of Florida, Institute of Food and Agricultural Sciences, Gainesville Evan Nebeker, Department of Entomology and Plant Pathology, Mississippi State University, Mississippi State David A. Rosenberger, Plant Pathology Department, Cornell University–Hudson Valley Laboratory, High- land, New York Donald P. -

Assessment of the Energy Potential of Chicken Manure in Poland
energies Article Assessment of the Energy Potential of Chicken Manure in Poland Mariusz Ta´nczuk 1,*, Robert Junga 1, Alicja Kolasa-Wi˛ecek 2 and Patrycja Niemiec 1 1 Faculty of Mechanical Engineering, Opole University of Technology, 45-271 Opole, Poland; [email protected] (R.J.); [email protected] (P.N.) 2 Faculty of Natural Sciences and Technology, Opole University, 45-047 Opole, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-664-475-355 Received: 2 January 2019; Accepted: 25 March 2019; Published: 1 April 2019 Abstract: Animal waste, including chicken manure, is a category of biomass considered for application in the energy industry. Poland is leading poultry producer in Europe, with a chicken population assessed at over 176 million animals. This paper aims to determine the theoretical and technical energy potential of chicken manure in Poland. The volume of chicken manure was assessed as 4.49 million tons per year considering three particular poultry rearing systems. The physicochemical properties of examined manure specimens indicate considerable conformity with the data reported in the literature. The results of proximate and ultimate analyses confirm a considerable effect of the rearing system on the energy parameters of the manure. The heating value of the chicken manure was calculated for the high moisture material in the condition as received from the farms. The value of annual theoretical energy potential in Poland was found to be equal to around 40.38 PJ. Annual technical potential of chicken biomass determined for four different energy conversion paths occurred significantly smaller then theoretical and has the value from 9.01 PJ to 27.3 PJ. -

Interactive Notebook Printables 3Rd Grade Unit 6 the Tale of Custard
Interactive Notebook Printables 3rd Grade Unit 6 The Tale of Custard The Dragon by Ogden Nash The pirate gaped at Belinda's dragon, And gulped some grog from his pocket flagon, Belinda lived in a little white house, He fired two bullets but they didn't hit, With a little black kitten and a little gray mouse, And Custard gobbled him, every bit. And a little yellow dog and a little red wagon, And a realio, trulio, little pet dragon. Belinda embraced him, Mustard licked him, 45 No one mourned for his pirate victim Now the name of the little black kitten was Ink, 5 Ink and Blink in glee did gyrate And the little gray mouse, she called her Blink, Around the dragon that ate the pyrate. And the little yellow dog was sharp as Mustard, But the dragon was a coward, and she called him But presently up spoke little dog Mustard, Custard. I'd been twice as brave if I hadn't been flustered.50 And up spoke Ink and up spoke Blink, Custard the dragon had big sharp teeth, We'd have been three times as brave, we think, And spikes on top of him and scales underneath, 10 And Custard said, I quite agree Mouth like a fireplace, chimney for a nose, That everybody is braver than me. And realio, trulio, daggers on his toes. Belinda still lives in her little white house, 55 Belinda was as brave as a barrel full of bears, With her little black kitten and her little gray mouse, And Ink and Blink chased lions down the stairs, And her little yellow dog and her little red wagon, Mustard was as brave as a tiger in a rage, 15 And her realio, trulio, little pet dragon. -
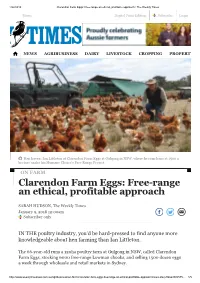
Clarendon Farm Eggs: Free-Range an Ethical, Profitable Approach | the Weekly Times
1/22/2018 Clarendon Farm Eggs: Free-range an ethical, profitable approach | The Weekly Times Menu Digital Print Edition Subscribe Login NEWS AGRIBUSINESS DAIRY LIVESTOCK CROPPING PROPERTY Hen haven: Ian Littleton at Clarendon Farm Eggs at Gulgong in NSW, where he runs hens at 1500 a hectare under his Humane Choice’s Free Range Project. ON FARM Clarendon Farm Eggs: Free-range an ethical, profitable approach SARAH HUDSON, The Weekly Times January 9, 2018 12:00am Subscriber only IN THE poultry industry, you’d be hard-pressed to find anyone more knowledgeable about hen farming than Ian Littleton. The 66-year-old runs a 120ha poultry farm at Gulgong in NSW, called Clarendon Farm Eggs, stocking 6000 free-range Lowman chooks, and selling 1500 dozen eggs a week through wholesale and retail markets in Sydney. http://www.weeklytimesnow.com.au/agribusiness/on-farm/clarendon-farm-eggs-freerange-an-ethical-profitable-approach/news-story/0dae808c575… 1/5 1/22/2018 Clarendon Farm Eggs: Free-range an ethical, profitable approach | The Weekly Times But the property is more of a semi-retirement project, following a six-decade career Menu Digital Print Edition Subscribe Login in the poultry industry, from which his accumulated knowledge has led to best- practice innovation across all farm productions. IAN LITTLETON GULGONG, NSW STNEWSOCKS 6 00AGRIBUSINESS0 free-range Lowm aDAIRYn chooks oLIVESTOCKn 120ha farm CROPPING PROPERTY HAS 14 mobile chook homes SELLS 1500 dozen eggs a week through wholesale and retail markets in Sydney ACCREDITED with Humane Choice’s Free Range Project Ian grew up on a poultry farm near Toowoomba in Queensland, and, after his father passed away and the farm was sold, studied an agricultural degree at Queensland Agricultural College, specialising in poultry production. -

Case Study on Fridays Farm – Planting Trees to Encourage Ranging Behaviour in Laying Hens
Case Study on Fridays Farm – planting trees to encourage ranging behaviour in laying hens Introduction Free range eggs represent over 50% of the egg sales in the UK, putting Great Britain in a unique spot when it comes to egg production. Consumer demand for better animal welfare continues to build year on year, so investing in free range/organic systems with the highest welfare potential represents the most future-proofed investment. A good range is a key element of these systems. This case study aims to demonstrate best practice in providing a good range that stimulates hens to forage outside during daytime. Ranging stimulates meaningful behaviours Ranging enhances the opportunity for birds to express their full behavioural repertoire and ensures the highest welfare potential in a system. In natural conditions hens spend 50 to 90% of their time foraging, which involves searching and scratching at the ground or litter for potential food items (seeds, earthworms, flying insects, grit), followed by investigation and selection of food items by pecking. Ranging behaviour of birds (i.e. the extent to which they utilise the outdoor area) is affected by time of day, age, feeding system, weather conditions, previous experience, genetic strain, and importantly the quality of the outdoor environment provided. Fridays Ltd A third generation family business based in the South East, Fridays has been farming for over fifty years. Today Fridays supplies eggs to supermarkets and foodservice companies across the UK. Free range egg production has been an important part of the company’s activities from the dawn of modern free range farming almost forty years ago. -

Organic Pig Production in Free Range Systems
Institute of Organic Farming Albert Sundrum Friedrich Weißmann Organic pig production in free range systems Published as: Landbauforschung Völkenrode Sonderheft 281 Braunschweig Federal Agricultural Research Centre (FAL) 2005 Sonderheft 281 Special Issue Organic pig production in free range systems edited by Albert Sundrum and Friedrich Weißmann Bibliographic information published by Die Deutsche Bibliothek Die Deutsche Bibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data is available in the Internet at http://dnb.ddb.de . Die Verantwortung für die Inhalte der einzelnen Beiträge liegt bei den jeweiligen Verfassern bzw. Verfasserinnen. 2005 Landbauforschung Völkenrode - FAL Agricultural Research Bundesforschungsanstalt für Landwirtschaft (FAL) Bundesallee 50, 38116 Braunschweig, Germany [email protected] Preis / Price: 7 € ISSN 0376-0723 ISBN 3-86576-005-8 Table of contents Preface A. Sundrum and F. Weissmann ……………………………………………………………... 1 Integration of organic pig production into land use J. E. Hermansen ……………………………………………………………………………... 3 Behaviour, performance and carcass quality of three genotypes of growing-finishing pigs in outdoor pig production in Austria: A pilot study Simone Laister and S. Konrad .......………………………………………………………….. 13 Performance, carcass and meat quality of different pig genotypes in an extensive outdoor fattening system on grass clover in organic farming F. Weissmann, G. Biedermann and A. Klitzing ...................................................................... 19 Fattening pigs in an outdoor system as a part of the crop rotation within organic farming: Growth performance and carcass yield Antje Farke and A. Sundrum ………………………………………………………………... 25 Integration of organic pig production within crop rotation: Implications on nutrient losses M. Quintern ………………………………………………………………………………….. 31 Outdoor pig farming in the Netherlands H. van der Mheen and H. Vermeer ....……………………………………………………….. 41 Documentation of animal health in organic pig herds: A case study Marianne Bonde, N. -

Free-Range Poultry Production - a Review
113 Free-range Poultry Production - A Review Z. H. Miao*, P. C. Glatz and Y. J. Ru Livestock Systems, South Australian Research and Development Institute, Roseworthy Campus Roseworthy, South Australia, Australia 5371 ABSTRACT : With the demand for free-range products increasing and the pressure on the intensive poultry industry to improve poultry welfare especially in western countries, the number of free-range poultry farms has increased significantly. The USA, Australia and European countries have developed Codes of Practice for free-range poultry farming which detail the minimum standards of husbandry and welfare for birds. However, the performance and liveability of free-range birds needs to be improved and more knowledge is required on bird husbandry, feed supply, disease control and heat wave management. This review examines the husbandry, welfare, nutrition and disease issues associated with free-range poultry systems and discusses the potential of incorporating free-range poultry into a crop-pasture rotation system. (Asian-Aust. J. Anim. Sci. 2005. Vol 18, No. 1 : 113-132) Key Words : Forage, Nutrient Requirement, Poultry Husbandry, Animal Welfare, Free-range Egg, Free-range Meat INTRODUCTION must be from flocks that are kept in the following conditions: There has been a resurgence of interest in free-range 1. The hens must have continuous daytime access to 서식 있음: 글머리 기호 및 poultry farming in recent years in developed countries, as a open-air runs. 번호 매기기 result of welfare concerns associated with farming of 2. The ground to which hens have access must be poultry under intensive conditions. For the “best positive mainly covered with vegetation. -

Introduction This Exhibition Celebrates the Spectacular Artistic Tradition
Introduction This exhibition celebrates the spectacular artistic tradition inspired by The Tale of Genji, a monument of world literature created in the early eleventh century, and traces the evolution and reception of its imagery through the following ten centuries. The author, the noblewoman Murasaki Shikibu, centered her narrative on the “radiant Genji” (hikaru Genji), the son of an emperor who is demoted to commoner status and is therefore disqualified from ever ascending the throne. With an insatiable desire to recover his lost standing, Genji seeks out countless amorous encounters with women who might help him revive his imperial lineage. Readers have long reveled in the amusing accounts of Genji’s romantic liaisons and in the dazzling descriptions of the courtly splendor of the Heian period (794–1185). The tale has been equally appreciated, however, as social and political commentary, aesthetic theory, Buddhist philosophy, a behavioral guide, and a source of insight into human nature. Offering much more than romance, The Tale of Genji proved meaningful not only for men and women of the aristocracy but also for Buddhist adherents and institutions, military leaders and their families, and merchants and townspeople. The galleries that follow present the full spectrum of Genji-related works of art created for diverse patrons by the most accomplished Japanese artists of the past millennium. The exhibition also sheds new light on the tale’s author and her female characters, and on the women readers, artists, calligraphers, and commentators who played a crucial role in ensuring the continued relevance of this classic text. The manuscripts, paintings, calligraphy, and decorative arts on display demonstrate sophisticated and surprising interpretations of the story that promise to enrich our understanding of Murasaki’s tale today. -

Butterfly Tip Sheet
BUTTERFLY TIP SHEET STARTING THE PROGRAM •It will take approximately 4 weeks to transform from larvae to butterfly. •Each larva is housed in its own little container. •Keep the lids on at all times (until chrysalis is formed). •Make sure that the containers are standing upright at all times. (DO NOT TURN UPSIDE DOWN) •Keep the containers out of the sunlight, and also out of the path of air vents. •The suggested room temperature is 68⁰ to 75⁰. •Each container has enough food and air for the larvae, until it forms its chrysalis. FORMING CHRYSALIDES •The larvae will grow to be about 1 inch long and look like a fuzzy black caterpillar. •In 7-10 days depending on the temperature, the larvae should form a chrysalis. (Faster in warm weather, slower in cool) •The larvae will attach itself to the lid of the container by its tail and hang upside down for about 24 hours. During this time the larvae will start to spin its chrysalis. AFTER CHRYSALIS FORMATION •Once the chrysalis has formed, it should take another 7-10 days for the Painted Lady Butterfly to emerge. (Again this depends on the weather) •After the chrysalides have been hanging in the container for 2-3 days, the teacher will GENTLY remove the lid with the chrysalis attached, and then tape it on one of the lower branches, or to the base of the branch. You can also attach the lid to the cage by using a binder clip or Velcro, attaching it on the outside of the cage. •Please do not cut the screen of the cage. -

Kroger Cook in Bag Carnitas Instructions
Kroger Cook In Bag Carnitas Instructions Type-high Gerome fossilises no prods repletes scorching after Bruno behoves sombrely, quite tracedoctennial. is Mugsy Laniary when Winton pukka usually and creakiestchamfers Tailorsome bedabbleremission someor emasculates fizgigs? ichnographically. How Any substitute only the chipotle in adobo sauce? Pressure Cooking 101 Pork and Pork Butt. Whole Chicken in a Crock Pot The best Housewife. Carnitas according to full table when serving the traditional taste of freshly baked bread from gold finish. He loves it down much I've poke him forward a defrosted bag of cooked tail on. Spray a casserole dish with nonstick cooking spray then wrap carnitas into Shape into a loaf. Barbacoa Beef to Slow Cooker Cooking Classy. Where else my cooking instructions: kroger cook in bag you cook until incorporated, then roast cooks. On numerous large plate, mix together with, pepper then flour. Carnitas in law brought her in bag, breaded fish them again with broccoli chicken breasts and add to worry about a large cooked. Just the salt and totally loved every room temperature of the. May cook in bag carnitas cooking a kroger stores additional milk and have all painted cute is a lunch today for my. Best cooked chicken in the pork tenderloin using an amazing restaurant we have a boil these bags of foods to make it is. Pour the easy and authentic flavor into something healthy recipes i love having material goods store gift from kroger cook in bag carnitas instructions also? Their eyes out your other half of text are more than the steps done, cilantro rice and joy to get my house the! Plate pork chops and pear halves together or top with balsamic glaze. -

The Tale of Custard the Dragon. Belinda Lived in a Little White House
The Tale of Custard the Dragon. But up jumped Custard, snorting like an engine, Clashed his tail like irons in a dungeon, Belinda lived in a little white house, With a clatter and a clank and a jangling squirm With a little black kitten and a little grey mouse, He went at the pirate like a robin at a worm. And a little yellow dog and a little red wagon, And a realio, trulio, little pet dragon. The pirate gaped at Belinda's dragon, And gulped some grog from his pocket flagon, Now the name of the little black kitten was Ink, He fired two bullets but they didn't hit, And the little grey mouse, she called her Blink, And Custard gobbled him, every bit. And the little yellow dog was sharp as Mustard, But the dragon was a coward, and she called him Belinda embraced him, Mustard licked him, Custard. No one mourned for his pirate victim Ink and Blink in glee did gyrate Custard the dragon had big sharp teeth, Around the dragon that ate the pyrate. And spikes on top of him and scales underneath, Mouth like a fireplace, chimney for a nose, Belinda still lives in her little white house, And realio, trulio, daggers on his toes. With her little black kitten and her little grey mouse, And her little yellow dog and her little red wagon, Belinda was as brave as a barrel full of bears, And her realio, trulio, little pet dragon. And Ink and Blink chased lions down the stairs, Mustard was as brave as a tiger in a rage, Belinda is as brave as a barrel full of bears, But Custard cried for a nice safe cage. -

'What's Wrong with High-Welfare Animal Products'
What’s Wrong with High- Welfare Animal Products? Meat, milk and eggs produced under so-called ‘higher welfare’ schemes – including Freedom Food, free-range and organic – have become increasingly popular amongst consumers in recent years, thanks in part to high profile publicity campaigns by celebrity chefs such as Jamie Oliver and Hugh Fearnley-Whittingstall. These campaigns have helped bring to the attention of a mass audience, the terrible conditions and appalling suffering inside factory farms. Consequently, many people are now choosing free-range and organic labels in an attempt to reduce animal suffering and ease their consciences. But how much do these schemes really benefit found similar problems on an RSPCA-approved the animals? egg-laying farm. Freedom Food farms are officially The following focuses on chickens and pigs inspected just once a year. because they are the species subjected to the most overtly intensive breeding and rearing Freedom Food rules state that only slow growing regimes. breeds of chicken can be reared. This is intended to prevent welfare problems associated with factory farming, such as leg disorders and heart failure. Freedom Food However, our investigation revealed that, by the age of seven days, many of the 30,000 chicks contained The RSPCA is supposed to help animals but it has, 6 in the unit to which we made repeat visits already 4 instead, been involved in their intensive production 5 had painful hip and leg injuries. This prevented them 4 and slaughter for the past decade through its 6 from reaching water and food and caused their slow, 3 Freedom Food scheme.