Promise Spring 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CNN Communications Press Contacts Press
CNN Communications Press Contacts Allison Gollust, EVP, & Chief Marketing Officer, CNN Worldwide [email protected] ___________________________________ CNN/U.S. Communications Barbara Levin, Vice President ([email protected]; @ blevinCNN) CNN Digital Worldwide, Great Big Story & Beme News Communications Matt Dornic, Vice President ([email protected], @mdornic) HLN Communications Alison Rudnick, Vice President ([email protected], @arudnickHLN) ___________________________________ Press Representatives (alphabetical order): Heather Brown, Senior Press Manager ([email protected], @hlaurenbrown) CNN Original Series: The History of Comedy, United Shades of America with W. Kamau Bell, This is Life with Lisa Ling, The Nineties, Declassified: Untold Stories of American Spies, Finding Jesus, The Radical Story of Patty Hearst Blair Cofield, Publicist ([email protected], @ blaircofield) CNN Newsroom with Fredricka Whitfield New Day Weekend with Christi Paul and Victor Blackwell Smerconish CNN Newsroom Weekend with Ana Cabrera CNN Atlanta, Miami and Dallas Bureaus and correspondents Breaking News Lauren Cone, Senior Press Manager ([email protected], @lconeCNN) CNN International programming and anchors CNNI correspondents CNN Newsroom with Isha Sesay and John Vause Richard Quest Jennifer Dargan, Director ([email protected]) CNN Films and CNN Films Presents Fareed Zakaria GPS Pam Gomez, Manager ([email protected], @pamelamgomez) Erin Burnett Outfront CNN Newsroom with Brooke Baldwin Poppy -

2 0 1 6 I N V E S T I T U
2016 INVESTITURE 2016 • MICHIGAN STATE UNIVERSITY MICHIGAN STATE 2016 INVESTITURE MICHIGAN STATE UNIVERSITY October 28, 2016 Michigan State University Auditorium A FORCE FOR CREATIVITY, DISCOVERY, AND LEARNING. Michigan State University has a legacy of being a dynamic, collaborative academic environment that prospective students and faculty are eager to join. We know that this distinctive environment is poised to generate a significant number of scientific breakthroughs as well as help students find their life’s work. It’s an environment where students, scholars, faculty, and researchers, through immeasurable creativity, discovery, and learning, will make seemingly impossible ideas possible and turn dreams into realities. Through the Empower Extraordinary campaign Michigan State University seeks to establish 100 new endowed chairs and fund important academic programs in order to retain and attract great thinkers and mentors. 1 The MSU INVESTITURE The Michigan State University investiture of 2016 celebrates MSU’s commitment to academic excellence and innovation by bestowing faculty recognition at the university level to those who hold endowed chair and professor positions. Today’s honorees, along with the entire faculty present at this inaugural event, are teachers and researchers whose work helps MSU to continue raising the bar on what we can achieve. The faculty who receive medallions today were appointed to positions funded during Empower Extraordinary, the campaign for MSU. As of October 1, 2016, 59 new endowed faculty positions have been created since the start of the campaign. 2 MSU BOARD OF TRUSTEES Joel I. Ferguson, Chairman Mitch Lyons, Vice Chairman Brian Breslin Dianne Byrum Melanie Foster Brian Mosallam George Perles Diann Woodard President Lou Anna K. -

Speaker Listing
SPEAKER LISTING 2020-21 2010-11 Harrison Hickman ’75 | Peniel Joseph Majora Carter | David Brooks | President Bill Clinton John Avlon & Margaret Hoover (w/Mark Updegrove) Jeannette Walls | Jean-Michel Cousteau Ian Bremmer | Sally Field (w/Pat Mitchell) Paul Nicklen | Theresa May | Colson Whitehead 2009-10 Garry Trudeau | Yo-Yo Ma | Paul Krugman 2019-20 Anna Deavere Smith | David Gregory Laura Bush | Stephen Breyer Doris Kearns Goodwin (w/Mark Updegrove) 2008-09 Khaled Hosseini | Christiane Amanpour & James Rubin 2018-19 Sir Salman Rushdie | Anthony Bourdain @ DPAC Karl Rove & David Axelrod | Kareem Abdul-Jabbar Anna Quindlen Julia Gillard | Dr. Paul Farmer | Diana Nyad 2007-08 2017-18 Kathleen Turner, Louis Gossett Jr. & Jane Seymour Joe & Jill Biden | Lisa Genova | Leslie Odom Jr. Isabel Allende | J.C. Watts | Bob Woodward Reza Aslan | Ted Koppel | Brandon Stanton 2006-07 2016-17 Mary Robinson | David McCullough | Toni Morrison Michael Pollan | Mark & Scott Kelly | Amal Clooney Neil deGrasse Tyson | Bryan Stevenson | Alan Alda 2005-06 Karen Armstrong | Desmond Tutu | Bill Moyers 2015-16 *Garrison Keillor (2 shows) Robin Wright | Atul Gawande | Jon Meacham 2004 - 05 George Takei | Malcolm Gladwell Cokie Roberts | Mikhail Gorbachev | Mary Pipher 2014-15 Michael Beschloss Ron Howard (w/Leonard Maltin) | Bill Bryson 2003-04 Margaret Atwood (w/ Roger Rosenblatt) Dr. Sherwin Nuland | Edward Albee | Ken Burns Robert Reich | Anderson Cooper Sidney Poitier | George J. Mitchell 2013-14 2002-03 Robert Gates | Robert Ballard | Itzhak Perlman Ernest Gaines | Robert F. Kennedy Jr. Elizabeth Alexander | Steve Kroft and Lesley Stahl 2001-02 2012-13 Madeleine Albright | Oscar Arias and Ralph Nader Tina Brown | Tom Brokaw | Geoffrey Canada Bill Bradley, Jeb Bush and Gwen Ifill 2000-01 Thomas Friedman Doris Kearns Goodwin | Jack Miles | Bill Bradley Neil deGrasse Tyson 2011-12 Tony Blair | Twyla Tharp | Sanjay Gupta 1999 *David McCullough @ Reynolds Auditorium Colin Powell Ken Burns | Fareed Zakaria 1996 Thomas Friedman. -

PLAYBOY INTERVIEW: SANJAY GUPTA a Candid Conversation with TV’S Most Respected Doctor About Staying Healthy, Avoiding Bogus Medical Advice and the Upside to Marijuana
PLAYBOY INTERVIEW: SANJAY GUPTA A candid conversation with TV’s most respected doctor about staying healthy, avoiding bogus medical advice and the upside to marijuana Yes, there is a doctor in the house. Whether appears on 60 Minutes. Since that pace is Mondays, many Fridays and often on it’s mass injuries in an earthquake, a celeb- too breezy, Gupta writes novels, competes in Thursdays too. But I like the balance. rity cancer scare or the war on obesity, Dr. triathlons (before his first race, at the age of My job at the hospital gives meaning to Sanjay Gupta is the source millions rely on 40, he taught himself to swim by watching my job on TV and vice versa. They’re for health information. CNN’s chief medi- YouTube videos) and spends quality time with similar challenges in many ways. They cal correspondent zips from war zones to his wife and three young daughters. both have the element of surprise. You virus hot zones and somehow finds time to Contributing writer David Hochman, who need to stay sharp and on your game, on practice brain surgery three days a week. last interviewed Bill Maher, met with Gupta top of the latest information, and both get When, say, a measles outbreak or a congres- during a week when Ebola was back in the your adrenaline going in a serious way. sional health care hearing makes headlines news. “I was astonished that Sanjay never PLAYBOY: Health news has become a at CNN headquarters in Atlanta, the multi- lost his focus or his cool even as the pres- media circus all its own in recent years. -
CNN.Com - Transcripts
CNN.com - Transcripts http://transcripts.cnn.com/TRANSCRIPTS/0702/01/ltm.02.html Member Center: Sign In | Register International Edition Search 1 of 11 2/1/07 9:42 PM CNN.com - Transcripts http://transcripts.cnn.com/TRANSCRIPTS/0702/01/ltm.02.html Home Page World Transcript Providers U.S. Weather Business Sports Return to Transcripts main page Analysis Politics AMERICAN MORNING Law Two Under Arrest for Boston Bomb Scare; New Tactics Technology Required in Iraq, Experts Say; Fight Against Childhood Science & Space Obesity Stirs Controversy Health Aired February 1, 2007 - 07:00 ET Entertainment THIS IS A RUSH TRANSCRIPT. THIS COPY MAY NOT Offbeat BE IN ITS FINAL FORM AND MAY BE UPDATED. Travel Education REYNOLDS WOLF, CNN METEOROLOGIST, AMERICAN MORNING: It will be mainly snow through the midmorning Special Reports hours, midday expect a transition going from the snow, switching over to sleet and then this afternoon there is Video the potential, the potential, for some freezing rain, which Autos could cause all kinds of issues with power lines as well as trees. I-Reports Right behind me, you can see, just a small oak tree, maybe 20, 30 feet tall or so. Some snow is picking up there. No ice as of yet which could cause damage. We are expecting that into the afternoon. Even farther behind, you can see a stretch of I-77 that has been pretreated Refinance and Save $1,000S yesterday, with a bit of a brine solution to help ward off $150,000 Mortgage for $483/month. Compare up to 4 free some of the ice and the possible snow that's going to be quotes.www.pickamortgage.com building up. -

History Early History
Cable News Network, almost always referred to by its initialism CNN, is a U.S. cable newsnetwork founded in 1980 by Ted Turner.[1][2] Upon its launch, CNN was the first network to provide 24-hour television news coverage,[3] and the first all-news television network in the United States.[4]While the news network has numerous affiliates, CNN primarily broadcasts from its headquarters at the CNN Center in Atlanta, the Time Warner Center in New York City, and studios in Washington, D.C. and Los Angeles. CNN is owned by parent company Time Warner, and the U.S. news network is a division of the Turner Broadcasting System.[5] CNN is sometimes referred to as CNN/U.S. to distinguish the North American channel from its international counterpart, CNN International. As of June 2008, CNN is available in over 93 million U.S. households.[6] Broadcast coverage extends to over 890,000 American hotel rooms,[6] and the U.S broadcast is also shown in Canada. Globally, CNN programming airs through CNN International, which can be seen by viewers in over 212 countries and territories.[7] In terms of regular viewers (Nielsen ratings), CNN rates as the United States' number two cable news network and has the most unique viewers (Nielsen Cume Ratings).[8] History Early history CNN's first broadcast with David Walkerand Lois Hart on June 1, 1980. Main article: History of CNN: 1980-2003 The Cable News Network was launched at 5:00 p.m. EST on Sunday June 1, 1980. After an introduction by Ted Turner, the husband and wife team of David Walker and Lois Hart anchored the first newscast.[9] Since its debut, CNN has expanded its reach to a number of cable and satellite television networks, several web sites, specialized closed-circuit networks (such as CNN Airport Network), and a radio network. -

Sanjay Gupta BIRTH DATE: October 23, 1969 in Novi, MI EDUCATION: M.D
POSITION: SURGEON GENERAL NOMINEE: Sanjay Gupta BIRTH DATE: October 23, 1969 in Novi, MI EDUCATION: M.D. 1992, University of Michigan Medical School B.A. in Medical Sciences, University of Michigan (Interflex 6-year program, combining pre-medical and medical school, accepted directly from high school) FAMILY: Married to Rebecca Olson Gupta; two daughters: Sage & Skye Clinton White House: 1997-1998 White House Fellow, special advisor to First Lady Hillary Clinton EXPERIENCE: 2001-present Chief medical correspondent for the health and medical unit at CNN 2001-present Assistant Professor of Neurosurgery, Emory University School of Medicine, Atlanta, GA Associate Chief of the Neurosurgery Service, Grady Memorial Hospital, Atlanta, GA 2000-2001 Partner of the Great Lakes Brain and Spine Institute 1998-2000 Fellow in neurosurgery, University of Tennessee Semmes-Murphy Clinic 1997-1998 White House Fellow 1992-1997 Residency in neurological surgery, University of Michigan Health System OTHER Was embedded with a Navy unit, the Devil Docs, during the 2003 Iraq invasion. In that time, he performed five brain surgeries Time magazine health column ON ABORTION and PRE-NATAL TESTING ”Basically, the reason the tests exist, to try to give young parents, or parents, an idea of how to proceed.” (Speaking on the benefits of prenatal testing) http://archives.cnn.com/TRANSCRIPTS/0312/16/ltm.05.html ON PHYSICIAN CONSCIENCE REGULATIONS and ABORTION Gupta has, for example, sharply criticized the regulations published by the Department of Health and Human Services. http://www.rhrealitycheck.org/blog/2009/01/06/dr-sanjay-gupta-tapped-surgeon-general “…it’s a bit of a slippery slope. -

Michigan State University Commencement Spring 2021
COMMENCEMENT CEREMONIES SPRING 2021 “Go forth with Spartan pride and confdence, and never lose the love for learning and the drive to make a diference that brought you to MSU.” Samuel L. Stanley Jr., M.D. President Michigan State University Photo above: an MSU entrance marker of brick and limestone, displaying our proud history as the nation’s pioneer land-grant university. On this—and other markers—is a band of alternating samara and acorns derived from maple and oak trees commonly found on campus. This pattern is repeated on the University Mace (see page 13). Inside Cover: Pattern of alternating samara and acorns. Michigan State University photos provided by University Communications. ENVIRONMENTAL TABLE OF CONTENTS STEWARDSHIP Mock Diplomas and the COMMENCEMENT Commencement Program Booklet 3-5 Commencement Ceremonies Commencement mock diplomas, 6 The Michigan State University Board of Trustees which are presented to degree 7 Michigan State University Mission Statement candidates at their commencement 8–10 Congratulatory Letters from the President, Provost, and Executive Vice President ceremonies, are 30% post-consumer 11 Michigan State University recycled content. The Commencement 12 Ceremony Lyrics program booklet is 100% post- 13 University Mace consumer recycled content. 14 Academic Attire Caps and Gowns BACCALAUREATE DEGREES Graduating seniors’ caps and gowns 16 Honors and master’s degrees’ caps and 17-20 College of Agriculture and Natural Resources gowns are made of post-consumer 21-22 Residential College in the Arts and Humanities recycled content; each cap and 23-25 College of Arts and Letters gown is made of a minimum of 26-34 The Eli Broad College of Business 23 plastic bottles. -

Experts Agree: It's Safe to Fly and America Is Open for Business
Experts Agree: It's Safe to Fly and America is Open for Business Government & Health Officials “We invite families and others to consider vacation here, “I think where these people are flying, it’s safe to fly. And large especially during spring break. We are still open for business portions of the world are very safe to fly. I think people are here in Orange County,” – Mayor Jerry Demings, Orlando, Florida going to be very impressed with what the airlines are doing.” – President Donald J. Trump Travel and Industry Voices “The American people deserve to know that, according to all of our experts, the risk to the average American of contracting the coronavirus remains low.” – Vice President Mike Pence For the most part [travelers] should be going on their spring breaks except for the hot spot areas that we know about. “I just want to echo again that the risk is low — the risk is low. I – Dr. John Torres, NBC Medical Correspondent encourage Americans to go about their life. That includes travel to California, Oregon, and the state of Washington.” – Director “Now is not the time to panic. Fear and panic undermine our Robert R. Redfield, M.D., Centers for Disease Control ability to contain the virus, minimize disruptions to daily life, and keep our economy humming along. Americans should “The risk to any average American is low, from the novel continue to monitor the situation but feel confident as they go coronavirus. The risk remains low. Thanks to the about their daily lives, head to work, conduct meetings, or drop unprecedented actions President Trump has taken and the their children off at school. -

6 Things You Didn't Know About Dr. Sanjay Gupta
October 11, 2013 (Online) 6 Things You Didn't Know About Dr. Sanjay Gupta Sanjay Gupta, photographed at Grady Memorial Hospital in Atlanta on Sept. 17.(Spencer Heyfron) http://www.parade.com/200470/leahrozen/7-things-you-didnt-know-about-dr-sanjay-gupta/ 345 Hudson Street, 16th Floor | New York, NY 10014 | Phone (646) 728-9500 | Fax (646) 728-9501 | www.EverydayHealth.com This weekend’s issue of Parade features CNN’s Sanjay Gupta on the cover. Here are six things he reveals that are not widely known about one of TV’s most popular doctors: He once wanted to change his name to Steve: As a boy growing up in the small community of Novi, Michigan, he encountered prejudice and bullying. Wanting to fit in, he proposed changing his name, inspired by Steve Austin of TV’s The Six Million Dollar Man. His mother talked him out of it. “I told him to be proud of his name, that one day everyone would know it because of the wonderful things he was going to do,” she recalls. He’s a Ford Motor Company loyalist: Gupta’s immigrant parents were engineers at Ford Motor Company, and he’s still loyal to the company. He drives a Ford Lincoln Navigator and a Jaguar bought when the British carmaker was owned by Ford. He’s much more than a doctor: In addition to his medical responsibilities (apart from his work at CNN and at Grady, he’s an assistant professor of neurosurgery at Emory University, contributes pieces to CBS’s 60 Minutes and does videos and blog posts for Everyday Health.com) Dr. -
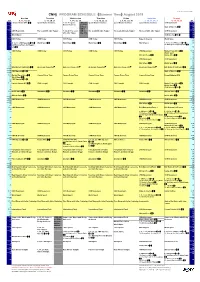
Cnnj PROGRAM SCHEDULE (Summer Time) August 2019
latest update:2019/6/25 16:30 CNNj PROGRAM SCHEDULE (Summer Time) August 2019 Monday Tuesday Wednesday Thursday Friday Saturday Sunday JST 5, 12, 19, 26 6, 13, 20, 27 7, 14, 21, 28 1, 8, 15, 22, 29 2, 9, 16, 23, 30 3, 10, 17, 24, 31 4, 11, 18, 25 ET 4:00 Fareed Zakaria GPS(R) Quest Means Business 7, 14, 28: Quest CNNj will Quest Means Business Quest Means Business Quest Means Business CNN Newsroom 15:00 :15 Means Business go off the :15 :30 air from State of America(R) :30 :45 0AM to :45 5:00 CNN Newsroom The Lead with Jake Tapper 7, 14, 28: The Lead 6AM on The Lead with Jake Tapper The Lead with Jake Tapper The Lead with Jake Tapper CNN Newsroom 16:00 :15 with Jake Tapper Aug. 21st. :15 :30 World Sport CNN Specials(TBA) :30 :45 :45 6:00 CNN Today CNN Today CNN Today CNN Today CNN Today State of America World Sport 17:00 :15 :15 :30 5, 12, 19: CNN Specials(TBA) World Sport(R) World Sport(R) World Sport(R) World Sport(R) World Sport 4, 11, 18, 25: CNN Specials(TBA) :30 26:Winning Post: An Elegant 28: Winning Post: An Elegant Life(R) :45 Life(R) :45 7:00 CNN Today CNN Today CNN Today CNN Today CNN Today CNN Newsroom Market Place Africa(R) 18:00 :15 World Sport :15 :30 African Voices(R) Inside Africa(R) :30 :45 :45 8:00 CNN Newsroom CNN Newsroom 19:00 :15 :15 :30 World Sport(R) World Sport(R) :30 :45 :45 9:00 Erin Burnett OutFront 2(R) Anderson Cooper360゜ Anderson Cooper360゜ Anderson Cooper360゜ Anderson Cooper360゜ Anderson Cooper360゜ Erin Burnett OutFront 1(R) 20:00 :15 :15 :30 CNN Specials(TBA) State of America(R) :30 :45 :45 10:00 Market -

2011 State of the News Media Report
Overview By Tom Rosenstiel and Amy Mitchell of the Project for Excellence in Journalism By several measures, the state of the American news media improved in 2010. After two dreadful years, most sectors of the industry saw revenue begin to recover. With some notable exceptions, cutbacks in newsrooms eased. And while still more talk than action, some experiments with new revenue models began to show signs of blossoming. Among the major sectors, only newspapers suffered continued revenue declines last year—an unmistakable sign that the structural economic problems facing newspapers are more severe than those of other media. When the final tallies are in, we estimate 1,000 to 1,500 more newsroom jobs will have been lost—meaning newspaper newsrooms are 30% smaller than in 2000. Beneath all this, however, a more fundamental challenge to journalism became clearer in the last year. The biggest issue ahead may not be lack of audience or even lack of new revenue experiments. It may be that in the digital realm the news industry is no longer in control of its own future. News organizations — old and new — still produce most of the content audiences consume. But each technological advance has added a new layer of complexity—and a new set of players—in connecting that content to consumers and advertisers. In the digital space, the organizations that produce the news increasingly rely on independent networks to sell their ads. They depend on aggregators (such as Google) and social networks (such as Facebook) to bring them a substantial portion of their audience. And now, as news consumption becomes more mobile, news companies must follow the rules of device makers (such as Apple) and software developers (Google again) to deliver their content.