Controls on Surface Water Quality in the River Clyde Catchment, Scotland UK, with Particular Reference to Chromium and Lead
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

South Lanarkshire Landscape Capacity Study for Wind Energy
South Lanarkshire Landscape Capacity Study for Wind Energy Report by IronsideFarrar 7948 / February 2016 South Lanarkshire Council Landscape Capacity Study for Wind Energy __________________________________________________________________________________________________________________________________________________________________________________________________________ CONTENTS 3.3 Landscape Designations 11 3.3.1 National Designations 11 EXECUTIVE SUMMARY Page No 3.3.2 Local and Regional Designations 11 1.0 INTRODUCTION 1 3.4 Other Designations 12 1.1 Background 1 3.4.1 Natural Heritage designations 12 1.2 National and Local Policy 2 3.4.2 Historic and cultural designations 12 1.3 The Capacity Study 2 3.4.3 Tourism and recreational interests 12 1.4 Landscape Capacity and Cumulative Impacts 2 4.0 VISUAL BASELINE 13 2.0 CUMULATIVE IMPACT AND CAPACITY METHODOLOGY 3 4.1 Visual Receptors 13 2.1 Purpose of Methodology 3 4.2 Visibility Analysis 15 2.2 Study Stages 3 4.2.1 Settlements 15 2.3 Scope of Assessment 4 4.2.2 Routes 15 2.3.1 Area Covered 4 4.2.3 Viewpoints 15 2.3.2 Wind Energy Development Types 4 4.2.4 Analysis of Visibility 15 2.3.3 Use of Geographical Information Systems 4 5.0 WIND TURBINES IN THE STUDY AREA 17 2.4 Landscape and Visual Baseline 4 5.1 Turbine Numbers and Distribution 17 2.5 Method for Determining Landscape Sensitivity and Capacity 4 5.1.1 Operating and Consented Wind Turbines 17 2.6 Defining Landscape Change and Cumulative Capacity 5 5.1.2 Proposed Windfarms and Turbines (at March 2015) 18 2.6.1 Cumulative Change -

FCC Environment Corporate Social Responsibility Report 2015 Contents 16 08 People Focus Who We Are
from waste to resource FCC Environment Corporate Social Responsibility Report 2015 Contents 16 08 People focus Who we are 32 Doing the right thing 04 06 Foreword from What we do 10 Paul Taylor Highlights and challenges 2 — 3 FCC Corporate Social Responsibility Report Introduction Doing the right thing 04 Foreword 33 Integrated Management System 06 What we do 34 Contributing to communities 08 Who we are Forward thinking Highlights and challenges 39 Introduction 11 Contract wins and renewals 41 Industry opinion 13 Infrastructure investments Appendix People focus 42 Appendix 1: 17 Health and safety Waste management methods 20 Equality and diversity 43 Appendix 2: 22 Competence Carbon emissions Management System 23 ABCD Awards Environmental commitment 25 Regulatory compliance 26 Reducing our energy use 28 Land restoration 30 Energy crops 24 Environmental commitment 38 Forward thinking Foreword As one of the UK’s largest waste management and recycling businesses, our corporate social responsibility is a meaningful gauge of FCC Environment’s sustainability. Over the last five years we have Will demand curtail, or will increased We look ahead to the future with transformed our safety culture, recycling to meet the demands of the enthusiasm, despite the uncertainties improved our standards of working, Circular Economy Package mean our we face: not least the British made strategic investments in European neighbours need more fuel referendum on EU membership; infrastructure, supported local to keep their lights on? the possible adoption of the communities, developed new revenue Circular Economy package; and In the meantime, we continue to look streams and significantly reduced its undetermined impacts on the ahead and provide leadership in the the environmental impacts of our marketplace. -

Rigside & Douglas Water
Rigside & Douglas Water Sustainable Travel Action Plan March 2019 1. Sustainable Travel Action Plan : RIGSIDE + DOUGLAS WATER March 2019 2. © Urbantu Ltd 14 West Terrace, South Queensferry EH30 9LL www.urbantu.design In Partnership with ARUP Scotstoun House, South Queensferry EH30 9SE www.arup.com We acknowledge the use of mapping and Imagery from the following sources: • Mapping data ©2018 Google (other data providers are acknowledged within the images credits on each respective image/graphic). • OS mapping - © Crown copyright and database rights 2018/19 Ordnance Survey 0100031673. • Understanding Scottish Places USP - Scottish Towns Partnership (STP) accessed 2018. • Data Shine Scotland Commute accessed at various time during 2018 Data Shine Scotland accessed at various time during 2018. This document has been quality checked and amended as follows: Version Date Description Created by Verified by Approved by V1 01/03/19 Draft issue for client review HH/SR/JC HH JC V2 08/03/19 Revised Draft issue for client HH/SR/JC HH JC review V3 22/03/19 Finalised Draft further to HH/SR/JC HH JC client comment V4 22/04/19 Appendicies removed as HH/SR/JC HH JC requested by RDT Sustainable Travel Action Plan : RIGSIDE + DOUGLAS WATER March 2019 3. Sustainable Travel Action Plan : RIGSIDE + DOUGLAS WATER March 2019 4. Contents P6 Introduction + Vision P8 Background P10 Methodology P12 Structuring Principles P13 Summary of Findings + Recommendations P17 Deliverability + Next Steps P19 Appendix A - Research Results P27 Appendix B - Baseline Sustainable Active Travel Assessment Sustainable Travel Action Plan : RIGSIDE + DOUGLAS WATER March 2019 5. Introduction The following Sustainable Travel Action Plan (STAP) has been developed to assist the communities of Rigside and Douglas Water to assess, evaluate and plan action(s) to increase active and sustainable travel; essentially encouraging people to walk, cycle and use public transport more and use private cars less. -

Douglas West & Dalquhandy DP Renewable Energy
Douglas West & Dalquhandy DP Renewable Energy Project NON-TECHNICAL SUMMARY July 2015 Non-Technical Summary Contents 1. Background 1 2. Purpose of the Proposed Development Environmental Statement (ES) 1 3. Availability of the Proposed Development ES 1 4. Representations to the Application 2 5. Site Location and Description 2 6. Site Selection and Design 4 7. Description of the Proposed Development 6 8. Programme 11 9. Consultation 12 10. Environmental Impact Assessment (EIA) 13 11. Benefits of the Proposed Development 21 12. Conclusion 22 DOUGLAS WEST & DALQUHANDY DP i NON-TECHNICAL SUMMARY RENEWABLE ENERGY PROJECT This page is intentionally blank. DOUGLAS WEST & DALQUHANDY DP ii NON-TECHNICAL SUMMARY RENEWABLE ENERGY PROJECT 1. Background 1.1 This document is a Non-Technical Summary of the Environmental Statement which accompanies an application by 3R Energy Solutions Limited (the Applicant), for consent under the Town and Country Planning (Scotland) Act 1997 (as amended), for the construction and operation of the Douglas West and Dalquhandy DP Renewable Energy Project (the Proposed Development), comprising 15 wind turbines and an associated Wood Fuel Drying Facility between Douglas and Coalburn, 11 km south west of Lanark, in rural South Lanarkshire. 2. Purpose of the Proposed Development Environmental Statement (ES) 2.1 Energised Environments Limited was appointed by the Applicant to undertake an Environmental Impact Assessment (EIA) of the Proposed Development in accordance with Schedule 2 of the Town and Country Planning (Environmental Impact Assessment) (Scotland) Regulation 2011. 2.2 The EIA process is reported in the ES, which describes the methods used to assess the beneficial and adverse environmental impacts predicted to result from the construction and operation of the Proposed Development. -

South Lanarkshire Core Paths Plan Adopted November 2012
South Lanarkshire Core Paths Plan Adopted November 2012 Core Paths list Core paths list South Lanarkshire UN/5783/1 Core Paths Plan November 2012 Rutherglen - Cambuslang Area Rutherglen - Cambuslang Area Map 16 Path CodeNorth Name Lanarkshire - Location Length (m) Path Code Name - Location LengthLarkhall-Law (m) CR/4/1 Rutherglen Bridge - Rutherglen Rd 360 CR/27/4 Mill Street 137 CR/5/1 Rutherglen Rd - Quay Rd 83 CR/29/1 Mill Street - Rutherglen Cemetery 274Key CR/5/2 Rutherglen Rd 313 CR/30/1 Mill Street - Rodger Drive Core233 Path CR/5/3 Glasgow Rd 99 CR/31/1 Kingsburn Grove-High Crosshill Aspirational530 Core Path Wider Network CR/5/4 Glasgow Rd / Camp Rd 543 CR/32/1 Cityford Burn - Kings Park Ave 182 HM/2280/1 Cross Boundary Link CR/9/1 Dalmarnock Br - Dalmarnock Junction 844 CR/33/1 Kingsheath Ave 460 HM/2470/1 Core Water Path CR/9/2 Dalmarnock Bridge 51 CR/34/1 Bankhead Road Water122 Access/Egress HM/2438/1 CR/13/1 Bridge Street path - Cambuslang footbridge 56 CR/35/1 Cityford Burn Aspirational164 Crossing CR/14/1 Clyde Walkway-NCR75 440 CR/36/1 Cityford Burn SLC276 Boundary Neighbour Boundary CR/15/1 Clyde Walkway - NCR 75 1026 CR/37/1 Landemer Drive 147 North Lanarkshire HM/2471/2 CR/15/2 NCR 75 865 CR/38/1 Landemer Drive Core Path93 Numbering CR/97 Land CR/15/3 Clyde Walkway - NCR 75 127 CR/39/1 Path back of Landemer Drive 63 UN/5775/1 Water CR/16/1 Clydeford Road 149 CR/40/1 Path back of Landemer Drive CL/5780/1 304 W1 Water Access/Egress Code CR/17/1 Clyde Walkway by Carmyle 221 CR/41/1 King's Park Avenue CL/3008/2 43 HM/2439/1 -

FCC Group 2020 Sustainability Report
FCC Group 2020 Sustainability Report FCC Group non-financial information report, in compliance with Law 11/2018 on non-financial information and diversity PROTECTING BIODIVERSITY........................................................................................................................ 80 8. Committed to the FCC Group Human Resources team ............................................................................................ 82 THE DNA OF THE HUMAN RESOURCES TEAM IN THE FCC GROUP ....................................................... 82 THE PEOPLE IN THE CENTRE: YOU_ .......................................................................................................... 83 HUMAN CAPITAL PROFILE ............................................................................................................................ 83 8.3.1 Diversity in the workforce ............................................................................................................................. 83 8.3.2 Organisational structure ............................................................................................................................... 85 8.3.3 Appreciation of job positions ........................................................................................................................ 85 8.3.4 Recruitment and dismissals ......................................................................................................................... 85 COMMITMENT TO TALENT ........................................................................................................................... -

17/11/2014 to : 21/11/2014
Enterprise Resources Planning and Building Standards Weekly List of Planning Applications List of planning applications registered by the Council for the week ending From : - 17/11/2014 To : 21/11/2014 Note to Members: Applications identified as 'Delegated' shall be dealt with under these powers unless more than 5 objections are received. In such cases the application will be referred to the appropriate area committee. Any queries on any of the applications contained in the list or requests to refer an application to committee should be directed to the area manager/team leader within 10 days of the week-ending date at the appropriate area office . A Member should only request that a team leader or manager consider referring a delegated application to committee if the Member still has concerns about an application after having discussed the matter with the team leader/manager. Note for Community Councils and members of the public: Further information If you wish further information on any application included in the list, please contact the case officer dealing with application . Officers can be contacted by phone on 0845 7406080 or by email [email protected]. Alternatively, you can view the application and associated documents on the Council's website at www.southlanarkshire.gov.uk Commenting on a planning application If you wish to comment on a planning application, you can do so by email [email protected], or in writing to Planning and Building Standards Services, Montrose House, 154 Montrose Crescent, Hamilton ML3 6LB or on the Council's website at www.southlanarkshire.gov.uk where you can submit comments directly through the application using the 'Search applications' button. -

This Is Document “Schedule 7 Part 7” Referred to in This Contract
3RD GENERATION TERM CONTRACT FOR MANAGEMENT AND MAINTENANCE OF THE SCOTTISH TRUNK ROAD NETWORK SOUTH WEST UNIT This is Document “Schedule 7 Part 7” referred to in this Contract SCOTTISH MINISTERS’ REQUIREMENTS SCHEDULE 7 PART 7 MANAGEMENT AND MAINTENANCE OF STRUCTURES CONTENTS Page No 1 INTRODUCTION 1 1.1 General 1 1.2 Definitions 1 2 MAINTENANCE MANAGEMENT 2 2.1 Management 2 2.2 Trunk Road Bridges Database (TRBDB) 2 2.3 Cyclic Maintenance Schedule 3 2.4 Maintenance and Operations Manuals and Health and Safety Files 3 3 INSPECTION REQUIREMENTS 4 3.1 General 4 3.2 Inspection Types 4 3.3 Weather Resistant Steel Bridge Monitoring 7 4 CYCLIC MAINTENANCE 7 4.1 General 7 4.2 Substructures and Superstructures 8 4.3 Expansion Joints 8 4.4 Drainage Systems 9 4.5 Parapets and Pedestrian Protection on Structures 9 4.6 Bearings and Bearing Shelves 9 4.7 Structures Over or Conveying Watercourses 10 4.8 Sign and/or Signal Gantries High Mast Lighting and Masts 10 4.9 Non-structural Items 11 4.10 Underpasses and Culverts also used by Pedestrians and Cyclists 11 4.11 Retaining Walls 12 5 REQUIREMENTS FOR STRUCTURAL MAINTENANCE 12 5.1 General 12 5.2 Maintenance Programme 13 6 STRUCTURAL ASSESSMENTS 14 6.1 General 14 6.2 Structural Assessment Process 14 6.3 Structural Assessments in Progress 14 7 MANAGEMENT OF SUB-STANDARD STRUCTURES AND STRUCTURES WITH KNOWN DEFECTS 15 7.1 General 15 7.2 Interim Measures and Monitoring 15 7.3 Replacement and Strengthening 16 8 ACCESS SYSTEMS 16 _________________________________________________________________________________________________________________ -

Planning Applications Index
North Lanarkshire Council Planning Applications for consideration of Planning and Transportation Committee Committee Date : April 2009 Ordnance Survey maps reproduced from Ordnance Survey with permission of HMSO Crown Copyright reserved APPLICATIONS FOR PLANNING AND TRANSPORTATION COMMITTEE l!jthApril 2009 Page Application No. Applicant Development/Locus Recommendation No 3 C/08/01023/FUL Airdrie North Ltd Construction of Waste to Grant C/o Agent Heat and Energy Plant, Recycling Facility, Access Road and Associated Works 50 S/08/01374/FUL Modern Housing Ltd Erection of Eight Flats and Grant One Residential Building Plot Land At Millbank Road Wishaw 60 S/09/00168/FUL Mr & Mrs Paris Conversion of Integral Refuse Garage to Habitable Room 17 Robert Wynd, Newmains Wishaw Application No: C/08/01023/FUL Date Registered: 22nd August 2008 Applicant: Airdrie North Ltd Clo Agent Agent James Barr Ltd 226 West George Street Glasgow G2 2LN Development: Construction of Waste to Heat and Energy Plant, Recycling Facility, Access Road and Associated Works Location: Land At Former Drumshangie OCCS Greengairs Road Green g a irs North Lanarkshire Ward: 007 Airdrie North Cllrs Cameron, McGuigan, Morgan and S Coyle Grid Reference: 277913668257 File Reference: C/P LIGWG 9OO/C M Site History: Refer to report Development Plan: Glasgow and The Clyde Valley Joint Structure Plan 2000 incorporatingthe Third Alterations 2006. Monklands District Local Plan 1991, Including Finalised First Alterations A, B & C September 1996 Contrary to Development Plan: Yes Consultations: Scottish Environment Protection Agency (Comments) Scottish Natural Heritage (Comments) Transport Scotland (No Objection) National Grid Gas Network (Comments) Scotland Gas Network (Comments) Scottish Power (Comments) Scottish Water (Comments) Historic Scotland (No objection) Architecture and Design Scotland (Comments) West Of Scotland Archaeology Service (Comments) Scottish Wildlife Trust (Comments) Royal Soc. -

Landscape Capacity Study for Wind Turbine Development in Glasgow and the Clyde Valley
Landscape Capacity Study for Wind Turbine Development in Glasgow and the Clyde Valley Overview Report Prepared by LUC for the Glasgow and the Clyde Valley Strategic Development Plan Authority September 2014 Project Title: Landscape Capacity Study for Wind Turbine Development in Glasgow and the Clyde Valley Client: Glasgow and the Clyde Valley Strategic Development Plan Authority In association with: Scottish Natural Heritage East Dunbartonshire Council East Renfrewshire Council Glasgow City Council Inverclyde Council North Lanarkshire Council Renfrewshire Council South Lanarkshire Council West Dunbartonshire Council Version Date Version Details Prepared by Checked by Approved by Principal 0.1 15 November Internal draft LUC PDM NJ 2013 0.2 22 November Interim draft for LUC PDM NJ 2013 discussion 1.0 25 March Draft LUC NJ NJ 2014 2.0 6 June 2014 Final LUC PDM NJ 3.0 11 September Revised LUC PDM NJ 2014 H:\1 Projects\58\5867 LIVE GCV wind farm study\B Project Working\REPORT\Overview report\GCV Report v3 20140911.docx Landscape Capacity Study for Wind Turbine Development in Glasgow and the Clyde Valley Overview Report Prepared by LUC for the Glasgow and the Clyde Valley Strategic Development Plan Authority September 2014 Planning & EIA LUC GLASGOW Offices also in: Land Use Consultants Ltd Registered in England Design 37 Otago Street London Registered number: 2549296 Landscape Planning Glasgow G12 8JJ Bristol Registered Office: Landscape Management Tel: 0141 334 9595 Edinburgh 43 Chalton Street Ecology Fax: 0141 334 7789 London NW1 -
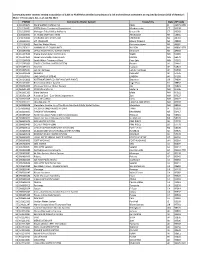
All Small-Sized Cwss That Have Certified Completion of Their RRA (Pdf)
Community water systems serving a population of 3,001 to 49,999 that certified completion of a risk and resilience assessment as required by Section 2013 of America's Water Infrastructure Act, as of July 30, 2021. PWSID Community Water System Town/City State ZIP Code 1 001570671 PACE WATER SYSTEM, INC. PACE FL 32571-0750 2 010106001 MPTN Water Treatment Department Mashantucket CT 06338 3 010109005 Mohegan Tribal Utility Authority Uncasville CT 06382 4 020000005 ST. REGIS MOHAWK TRIBE Akwesasne NY 13655 5 043740039 CHEROKEE WATER SYSTEM CHEROKEE NC 28719 6 055293201 MT. PLEASANT Mount Pleasant MI 48858 7 055293603 East Bay Water Works Peshawbestown MI 49682 8 055293611 HANNAHVILLE COMMUNITY WILSON MI 49896-9728 9 055293702 LITTLE RIVER TRIBAL WATER SYSTEM Manistee MI 49660 10 055294502 Prairie Island Indian Community Welch MN 55089 11 055294503 Lower Sioux Indian Community Morton MN 56270 12 055294506 South Water Treatment Plant Prior Lake MN 55372 13 055295003 SOUTH-CENTRAL WATER SYSTEM Bowler WI 54416 14 055295310 Giiwedin Hayward WI 54843 15 055295401 Lac du Flambeau Lac du Flambeau WI 54538 16 055295508 KESHENA KESHENA WI 54135 17 055295703 ONEIDA #1 OR SITE #1 ONEIDA WI 54155 18 061020808 POTTAWATOMIE CO. RWD #3 (DALE PLANT) Shawnee OK 74804 19 061620001 Reservation Water System Eagle Pass TX 78852 20 062004336 Chicksaw Winstar Water System Ada OK 74821 21 063501100 POJOAQUE SOUTH Santa Fe NM 87506 22 063501109 Isleta Eastside Isleta NM 87022 23 063501124 Pueblo of Zuni - Zuni Utility Department Zuni NM 87327 24 063503109 Isleta Shea Whiff Isleta NM 87022 25 063503111 LAGUNA VALLEY LAGUNA, NM 87026 NM 87007 26 063506008 Mescalero Apache Inn of the Mountain Gods Public Water System Mescalero NM 88340 27 070000003 SAC & FOX (MESKWAKI) IN IOWA TAMA IA 52339 28 083090091 TOWN OF BROWNING BROWNING MT 59417 29 083890023 Turtle Mountain Public Utilities Commission Belcourt ND 58316 30 083890025 Spirit Lake Water Management RWS St. -

Voices from the Grassroots
VOICES FROM THE GRASSROOTS Redressing the Balance: working towards environmental justice in Scotland VOICES FROM THE GRASSROOTS Redressing the Balance: working towards environmental justice in Scotland. Handbook 4 Sept 2003 ISBN: 1 901855 16 3 Written by the Agents for Environmental Justice and edited by Eurig Scandrett. Graphics design and coordination by Sarah de Mowbray. Cover picture: Colin Hattersley Printed on non-chlorine-bleached, 100% recycled paper by Alphagraphics, tel. 0131 316 1800 This handbook is supported by The Community Fund as part of the Agents for Environmental Justice Project Friends of the Earth Scotland 72 Newhaven Road, Edinburgh EH6 5QG Tel: 0131 554 9977; Fax: 0131 554 8656 E-mail: [email protected] Website: www.foe-scotland.org.uk FOE Scotland is a charity (SC003442) Contents page Chapter 1. Introduction 1.1 What is environmental justice? 4 1.2 Dialogue with struggle 5 Chapter2. Case Studies 7 2.1 Life in the Sacrificial Zone - Ann Coleman, Greengairs and Wattston 8 2.2 Shouting from the shore - Aaron Forsyth, Scoraig 12 2.3 Brick by brick - Kirsten Marshall, Dundyven, Coatbridge 16 2.4 Justice and Waste: Reflections from a Scottish Island - Terry Hegarty, Isle of Mull 18 2.5 Bypassing the System: Roads and power - Joan Higginson, Penicuik 22 2.6 Black resistance to a green revolution: Punjab and Edinburgh - Nahid Aslam, Edinburgh 26 2.7 Environmental Justice and the Fish Farming Industry - Victor Thomas, Shetland 29 2.8 Falkirk to Johannesburg - Sonia McLay, Falkirk 33 2.9 David & Goliath - Sue Fenton, Farr, Inverness-shire 36 2.10 Blaws the cloods heelster gowdie ower the Ben? - Rod Lovie, Keith Morayshire 40 2.11 A better environment than opencast - Andy Robinson, South Lanarkshire 44 Chapter 3.