Quantitative Chemical Analysis 7E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CO2, Hothouse and Snowball Earth
CO2, Hothouse and Snowball Earth Gareth E. Roberts Department of Mathematics and Computer Science College of the Holy Cross Worcester, MA, USA Mathematical Models MATH 303 Fall 2018 November 12 and 14, 2018 Roberts (Holy Cross) CO2, Hothouse and Snowball Earth Mathematical Models 1 / 42 Lecture Outline The Greenhouse Effect The Keeling Curve and the Earth’s climate history Consequences of Global Warming The long- and short-term carbon cycles and silicate weathering The Snowball Earth hypothesis Roberts (Holy Cross) CO2, Hothouse and Snowball Earth Mathematical Models 2 / 42 Chapter 1 Historical Overview of Climate Change Science Frequently Asked Question 1.3 What is the Greenhouse Effect? The Sun powers Earth’s climate, radiating energy at very short Earth’s natural greenhouse effect makes life as we know it pos- wavelengths, predominately in the visible or near-visible (e.g., ul- sible. However, human activities, primarily the burning of fossil traviolet) part of the spectrum. Roughly one-third of the solar fuels and clearing of forests, have greatly intensifi ed the natural energy that reaches the top of Earth’s atmosphere is refl ected di- greenhouse effect, causing global warming. rectly back to space. The remaining two-thirds is absorbed by the The two most abundant gases in the atmosphere, nitrogen surface and, to a lesser extent, by the atmosphere. To balance the (comprising 78% of the dry atmosphere) and oxygen (comprising absorbed incoming energy, the Earth must, on average, radiate the 21%), exert almost no greenhouse effect. Instead, the greenhouse same amount of energy back to space. Because the Earth is much effect comes from molecules that are more complex and much less colder than the Sun, it radiates at much longer wavelengths, pri- common. -

Keeling Curve: Result, Interpretation & Global Monitoring
International Journal for Empirical Education and Research Keeling Curve: Result, Interpretation & Global Monitoring Augustyn Ostrowski Faculty of Geography & Geology Jagiellonian University Email: [email protected] (Author of Correspondence) Poland Abstract The Keeling Curve is a graph of the accumulation of carbon dioxide in the Earth's atmosphere based on continuous measurements taken at the Mauna Loa Observatory on the island of Hawaii from 1958 to the present day. The curve is named for the scientist Charles David Keeling, who started the monitoring program and supervised it until his death in 2005. Keywords: Mauna Loa Measurements; Results and Interpretation; Global Monitoring; Ralph Keeling. ISSN Online: 2616-4833 ISSN Print: 2616-4817 35 1. Introduction Keeling's measurements showed the first significant evidence of rapidly increasing carbon dioxide levels in the atmosphere. According to Dr Naomi Oreskes, Professor of History of Science at Harvard University, the Keeling curve is one of the most important scientific works of the 20th century. Many scientists credit the Keeling curve with first bringing the world's attention to the current increase of carbon dioxide in the atmosphere. Prior to the 1950s, measurements of atmospheric carbon dioxide concentrations had been taken on an ad hoc basis at a variety of locations. In 1938, engineer and amateur meteorologist Guy Stewart Callendar compared datasets of atmospheric carbon dioxide from Kew in 1898-1901, which averaged 274 parts per million by volume (ppm), and from the eastern United States in 1936-1938, which averaged 310 ppmv, and concluded that carbon dioxide concentrations were rising due to anthropogenic emissions. However, Callendar's findings were not widely accepted by the scientific community due to the patchy nature of the measurements. -

How I Stave Off Despair As a Climate Scientist So Much Warming, So Many Dire Effects, So Little Action — Dave Reay Reveals YVONNE COOPER/UNIV
WORLD VIEW A personal take on events How I stave off despair as a climate scientist So much warming, so many dire effects, so little action — Dave Reay reveals YVONNE COOPER/UNIV. EDINBURGH COOPER/UNIV. YVONNE how dreams of soggy soil and seaweed keep him going. here’s a curve that is quietly plotting our performance as a behind my eyelids, that’s what helps me sleep. species. This curve is not a commodity price or a technology That, and a personal plot to pull a lifetime’s worth of carbon out of index. It has no agenda or steering committee. It is the Keeling the atmosphere. Tcurve. It is painfully consistent in its trajectory and brutally honest in The dream with which I’ve bored my family to distraction for the its graphical indictment of our society as one that stands ready to stand past 20 years is going truly ‘net zero’: paring down emissions to the by as islands submerge, cities burn and coasts flood. bare minimum, and then managing a chunk of land to try to sequester Established by Charles David Keeling in 1958, the curve records the remainder. how much carbon dioxide is in our atmosphere — fewer than 330 parts Last month, that dream came true. Years of saving, a large dollop per million then, more than 400 today. Each month for the past decade, of luck and an even larger loan made me and my wife the nervous my geeky addiction has been to scan the latest data. To search for some owners of 28 hectares of rough grassland and wild rocky shores in hint that ‘Stabilization Day’ will come: when global emissions and the west of Scotland. -

The Scripps Carbon Dioxide and Oxygen Programs Ralph F. Keeling Scripps Institution of Oceanography [email protected]
The Scripps carbon dioxide and oxygen programs Ralph F. Keeling Scripps Institution of Oceanography [email protected] The Scripps CO2 program was initiated in 1956 by Charles David Keeling and operated under his direction until his passing in 2005. It is currently being continued by Ralph F. Keeling, who also runs the parallel Scripps O2 program. The combined programs are sustaining the longest continuous measurements of changes in atmospheric carbon dioxide concentration, the isotopes of CO2, the atmospheric O2 concentration (as O2/N2 ratio). The program involves flask collections made through cooperative programs with field stations at roughly a dozen stations around the world. The emphasis in the program is in the detection of global and hemispheric trends in these species. The measurements are pertinent to assessing global and hemispheric sources and sinks of CO2 and their changes from year to year. The records are also relevant source/sink estimates on finer spatial scales, and are among the first places to look for evidence of climate feedbacks or other "surprises" in the records. For more than decade, the program has been archiving CO2 from air samples in sealed glass ampoules for retrospective analysis. As an example of an application, these archives has recently allowed the generation of time series of the radiocarbon content of atmospheric CO2, which is relevant for assessing the distribution of carbon between reservoirs and rates of fossil-fuel burning. The O2 program has also expanded recently to include measurements of the Ar concentration (as Ar/N2 ratio). This ratio varies as the ocean warms and cools due to thermally-driven ingassing and outgassing of Ar and N2 by the oceans. -

A Rational Discussion of Climate Change: the Science, the Evidence, the Response
A RATIONAL DISCUSSION OF CLIMATE CHANGE: THE SCIENCE, THE EVIDENCE, THE RESPONSE HEARING BEFORE THE SUBCOMMITTEE ON ENERGY AND ENVIRONMENT COMMITTEE ON SCIENCE AND TECHNOLOGY HOUSE OF REPRESENTATIVES ONE HUNDRED ELEVENTH CONGRESS SECOND SESSION NOVEMBER 17, 2010 Serial No. 111–114 Printed for the use of the Committee on Science and Technology ( Available via the World Wide Web: http://www.science.house.gov U.S. GOVERNMENT PRINTING OFFICE 62–618PDF WASHINGTON : 2010 For sale by the Superintendent of Documents, U.S. Government Printing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 COMMITTEE ON SCIENCE AND TECHNOLOGY HON. BART GORDON, Tennessee, Chair JERRY F. COSTELLO, Illinois RALPH M. HALL, Texas EDDIE BERNICE JOHNSON, Texas F. JAMES SENSENBRENNER JR., LYNN C. WOOLSEY, California Wisconsin DAVID WU, Oregon LAMAR S. SMITH, Texas BRIAN BAIRD, Washington DANA ROHRABACHER, California BRAD MILLER, North Carolina ROSCOE G. BARTLETT, Maryland DANIEL LIPINSKI, Illinois VERNON J. EHLERS, Michigan GABRIELLE GIFFORDS, Arizona FRANK D. LUCAS, Oklahoma DONNA F. EDWARDS, Maryland JUDY BIGGERT, Illinois MARCIA L. FUDGE, Ohio W. TODD AKIN, Missouri BEN R. LUJA´ N, New Mexico RANDY NEUGEBAUER, Texas PAUL D. TONKO, New York BOB INGLIS, South Carolina STEVEN R. ROTHMAN, New Jersey MICHAEL T. MCCAUL, Texas JIM MATHESON, Utah MARIO DIAZ-BALART, Florida LINCOLN DAVIS, Tennessee BRIAN P. BILBRAY, California BEN CHANDLER, Kentucky ADRIAN SMITH, Nebraska RUSS CARNAHAN, Missouri PAUL C. BROUN, Georgia BARON P. HILL, Indiana PETE OLSON, Texas HARRY E. MITCHELL, Arizona CHARLES A. WILSON, Ohio KATHLEEN DAHLKEMPER, Pennsylvania ALAN GRAYSON, Florida SUZANNE M. -

Chapter 1 NOTES
NOTES Chapter 1 1. Emphasis added, quoted from An Inconvenient Truth: A Global Warning, di- rected by David Guggenheim and released by Paramount Classics in 2006. This film was an official selection of the 2006 Sundance Film Festival and the Cannes Film Festival, and won the Academy Award in 2007 for Best Documentary. The printed companion is Al Gore, An Inconvenient Truth: The Planetary Emergency of Global Warming and What We Can Do About It (New York: Rodale, 2006). Al Gore shared the 2007 Nobel Peace Prize with the Intergovernmental Panel on Climate Change (IPCC) for his leadership on global warming. 2. Bjorn Lomborg, e.g., emphasized the differences between Gore and the IPCC in “Ignore Gore—But Not His Nobel Friends,” The Sunday Telegraph (London) (11 November 2007), 24. On the scientific consensus in the United States, see Jane A. Leggett, Climate Change: Science and Policy Implications, CRS Report for Congress RL33849 (Washington, D.C.: Congressional Research Service, Updated 2 May 2007). On the scientific consensus in the international community, see the widely publi- cized assessments of the IPCC that are introduced below and summarized in later chapters. 3. The Framework Convention and related sources can be accessed at the Gate- way to the UN System’s Work on Climate Change, http://www.un.org/climatechange/ projects.shtml. 317 4. See the UNFCCC’s background information on the Kyoto Protocol, accessed 5 September 2007, at http://unfccc.int/kyoto_protocol/background/items/3145.php. See also Susan R. Fletcher and Larry Parker, Climate Change: The Kyoto Protocol and International Actions, CRS Report for Congress RL 33836 (Washington, D.C.: Congressional Research Service, Updated 8 June 2007). -

An Inconvenient Truth: Transcript
An Inconvenient Truth: Transcript From: http://forumpolitics.com/blogs/2007/03/17/an-inconvient-truth-transcript March 17, 2007 Introduction You look at that river gently flowing by. You notice the leaves rustling with the wind. You hear the birds; you hear the tree frogs. In the distance you hear a cow. You feel the grass. The mud gives a little bit on the river bank. It’s quiet; it’s peaceful. And all of a sudden, it’s a gear shift inside you. And it’s like taking a deep breath and going, “Oh yeah, I forgot about this.” Earth Rise This is the first picture of the Earth from space that any of us ever saw. It was taken on Christmas Eve 1968 during the Apollo 8 mission. More…In relatively comfortable boundaries… But we are filling up that thin shell of atmosphere with pollutants. I’m Al Gore. I used to be the next president of the United States. [ laughter and applause from audience ] I don’t find that particularly funny. I’ve been trying to tell this story for a long time and I feel as I’ve failed to get the message across. I was in politics for a long time. I’m proud of my services. (Mayor of New Orleans in background ). There are good people who are in politics who hold this at arm’s length because they acknowledge it and recognize it as a moral imperative to make big changes. And they lost radio contact when they went around to the dark side of the moon and there was inevitably some suspense. -

Enjoying the Great Outdoors Charles Keeling Loves the Great Outdoors
2 by John Leaf Visuals || Text Enjoying the Great Outdoors Charles Keeling loves the great outdoors. That poses a challenge. The year is 1953 and the post-World War II economy is booming. People across the nation are starting families and buying homes. They are equipping them with new technologies, such as dishwashers, frozen foods and televisions. The air is filled with a sense of scientific progress. Just out of chemistry graduate school in Illinois, Keeling is looking for a job. With a background in studying polymers (used to make plastics), he has plenty of opportunities. However, Keeling will not take any job that comes his way. In tune with the outdoors, he wants to find something on the West Coast. Keeling's advisor thinks he is a fool to turn down multiple East Coast jobs offering good pay. OPTIONAL THINK (A). Would you sacrifice good jobs for something you loved? What do you think are the chances Keeling will succeed? Keeling writes letters to Geology departments in the West, presenting his services as a chemist. Most politely decline. But Keeling gets two offers. He accepts a postdoctoral position at the California Institute of Technology in southern California. Although Keeling has some knowledge of geology he is inexperienced. He is unsure what research to pursue, so he is assigned to a project on extracting uranium from granites to use for nuclear power. The fit seems logical for a chemist like Keeling. His first task is to crush rocks for two weeks. The goal of the study interests Keeling enough, but rock crushing frankly doesn't inspire him. -

Inconvenient Truth Discussion Questions
Name: __________________________ Class: _____________________ Date: _____________________ Inconvenient Truth Discussion Questions Background 1. A quote often attributed to Mark Twain states, “What gets us into trouble is not what we don't know. It's what we know for sure that just ain't so." How does this relate to Pangaea and the Theory of Plate Tectonics? The idea that the continents fit together was rejected by many for along time, as the notion of entire land masses moving was simply too unbelievable. This, of course, turned out to be true. 2. The sun emits three main wavelengths of energy that reach the earth. Which is responsible for global warming? a. Infrared b. Ultraviolet c. Visible 3. A clip from the Futurama episode Crimes of the Hot is shown. At the end of the clip, global warming is “solved” by dropping larger and larger ice cubes into the ocean. a. How is this a metaphor for how global warming has been handled in the real world? The problem isn’t really being dealt with or given any urgency. We may be forced to come up with a last-minute solution. b. Assume that humanity had the technology to mine and transport large amounts of ice from other planets to the Earth. Why would this solution not actually work? Among other problems, as the ice in the ocean melted, it would cause sea levels to rise, creating a massive amount of flooding in coastal areas. Biology Teaching Resources http://www.aurumscience.com Page 1 4. A scientist named Charles David Keeling began recording daily carbon dioxide levels at an observatory in Mauna Loa, Hawaii in 1958. -

Tribute to Roger Revelle and His Contribution to Studies of Carbon Dioxide and Climate Change
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 8275–8279, August 1997 Colloquium Paper This paper was presented at a colloquium entitled ‘‘Carbon Dioxide and Climate Change,’’ organized by Charles D. Keeling, held November 13–15, 1995, at the National Academy of Sciences, Irvine, CA. Tribute to Roger Revelle and his contribution to studies of carbon dioxide and climate change WALTER H. MUNK Institute of Geophysics and Planetary Physics, University of California at San Diego, La Jolla, CA 92093-0225 I first came to Scripps Institution of Oceanography (SIO) in Harald Sverdrup was anxious for Roger to return to La Jolla the summer of 1939, after completing my junior year at the to succeed him as director of Scripps. Sverdrup (2) wrote, California Institute of Technology. Roger Revelle was 30 years ‘‘regardless of the capacity in which you return here, you are old, with the rank of instructor (long since abolished by the the logical man to take charge...ofthework at sea.’’ University of California), and a lieutenant junior grade in the And Roger (3) agreed: Naval Reserve. Roger invited me to come along on an Sverdrup’s support for me as successor is also based experiment to measure currents in the waters over the Cali- upon the fact that I am practically the only person fornia borderland. The standard tool was an Ekman Current available who has had extensive experience at sea, in Meter; for every 100 revolutions of a propeller, a 2-mm ball is particular in the organization and carrying out of expe- dropped into a compass box with 36 compartments, each corresponding to a 10° segment in current direction. -

Scripps Global Climate Change Pioneer to Receive Tyler Prize
Scripps Global Climate Change Pioneer To Receive Tyler Prize Charles David Keeling honored for world-leading research on carbon and climate March 30, 2005 Dora Dalton Charles David Keeling, a professor of oceanography at Scripps Institution of Oceanography at the University of California, San Diego, has been selected to receive the Tyler Prize for Environmental Achievement, which is awarded for accomplishments in environmental science, energy and medicine that confer great benefit upon mankind. Keeling and co-honoree Lonnie Thompson, University Professor of Geology at Ohio State University, will be formally awarded at a black-tie ceremony at the Four Seasons Hotel Los Angeles in Beverly Hills on April 8. The two will also give public lectures on April 7 at the Davidson Conference Center at the University of Southern California. Keeling, a world leader in research on the carbon cycle and the increase of carbon dioxide (CO2) in the atmosphere, known to influence the greenhouse effect, has been affiliated with Scripps since 1956. "Dave Keeling, more than anyone else, established the imminence of global warming as the most profound, enveloping and inclusive environmental challenge facing mankind," said Charles Kennel, director of Scripps Institution of Oceanography. "His research continues today with the same rigor and dedication that have characterized the past four decades. I can think of no individual who has made a more significant contribution to the modern science of global change research or to our understanding of the global carbon -
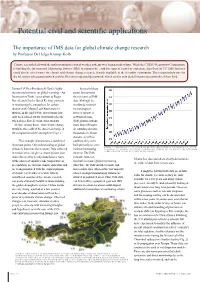
CTBTO Spectrum 2007 Article XIV Conference Special Edition (Issue
Potential civil and scientific applications The importance of IMS data for global climate change research by Professor Dr helga Kromp-Kolb Climate research deals with the analysis and projection of weather patterns over long periods of time. While the CTBTO Preparatory Commission is building the International Monitoring System (IMS) to monitor the earth for signs of a nuclear explosion, data from its 337 IMS facilities could also be of relevance for climate and climate change research, if made available to the scientific community. This is particularly true for the 60 station-infrasound network and the 80 station-radionuclide network, which are the only global monitoring networks of their kind. former uS Vice-President al gore’s highly Several of these recommended movie on global warming, “An points demonstrate Inconvenient Truth”, pays tribute to Roger the relevance of IMS Revelle and Charles David Keeling, pioneers data. Although the in monitoring the atmosphere for carbon worldwide national dioxide at the Mauna Loa Observatory in meteorological Hawaii. In the mid-1960s, observations had services operate a only been carried out for about half a decade network of some when Roger Revelle warned his students 5000 ground stations, – Al Gore among them – that climate change more than 600 upper would be the result of the observed change in air sounding stations, the composition of the atmosphere (Fig.1). thousands of climate stations, as well as This example demonstrates a number of satellites, they were important points. Our understanding of global built primarily to serve Figure : Atmospheric CO 2 concentrations (ppmV) DeriveD frOm aIr SamPleS collecteD climate is based on observations.