Volume 18(1), 123- 130, 2014
JOURNAL of Horticulture, Forestry and Biotechnology
Rhododendron ferrugineum L. and Rhododendron myrtifolium
Schott & Kotschy in habitats from Eastern Alps mountains and Carpathian Mountains
Căprar M.1,2, Cantor Maria2*, Szatmari P.1, Sicora C.1
1)Biological Research Center, Botanical Garden ,,Vasile Fati” Jibou, Parcului Street,no.14,455200 Jibou, Romania; 2)University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Faculty of Horticulture, Mănăștur Street, no 3-5,4000472 Cluj-Napoca, Romania;
* Corresponding author. Email: [email protected]
Abstract
This paper presents results of research carried on two species Key words of Rhododendron in habitats from different regions of Central and Eastern
Europe (Rhododendron ferrugineum and Rhododendron myrtifolium). It rhododendrons
species, presents the ecological requirements of each habitat, their spread, main plant habitats, plant communities, association and floristic composition based on the dominance of probative Carpathian Mountains, Alps species. A correlation is made between habitats from different classifications, Mountains but with the same features, mentioning EUNIS codes, Emerald, Natura 2000, Palaearctic Habitats and the European forest types. This paper presents information on the spread of two types of habitats containing Rhododendronfrom Europe, the environmental conditions in which they live and the accompanying species involved, more or less, in the composition of habitats. It describes the types of vegetation in the Alps (Austria) and the Carpathian Mountains (Romania). Vegetation was observed following the research in the field.
Knowledge of the habitats in which
Rhododendron species live becomes an important objective in order to protect these species that in most European countries are rare or endangered. The rhododendron habitats host a series of species that are rare and scientifically interesting, from witness of ice ages to survivors of Tertiary or unique endemic species. Conservation of these habitats will help perpetuate the species and better understand the functioning of ecosystems. of Australia, with over 200 species only in the island of New Guinea [2].
Rhododendron species vary in shape and height from miniature plants, repentis that forme pads under the action of external factors, as far as 30 m trees in mountain forests. Many species grow at high altitudes, over 900 m, some on rocks, some are epiphytic on tree branches. They occupy a wide variety of ecosystems, tropical mountain forests and alpine meadows at 4000 m altitude [19].
- The genus Rhododendron L. (in ancient Greek
- Flowers may be solitary or in up to 24
- "rhodon" the rose, and "dendron" meaning tree) [11] belongs to the family Ericaceae, a highly complex genre, totaling over 1000 species worldwide. According to Heywood there are around 700 species only in the region covering China, Tibet, Nepal, Assam (Northeast India) and Myanmar [12]. Woody species, some of which are evergreen, others deciduous, meet along the entire northern hemisphere. Most are found in temperate and cold regions with a high concentration of species in western China, the Himalayas and northeastern Myanmar (Burma) [16]. Due to unfavorable conditions some of them have adapted by losing leaves in winter most frequent seem in North America, China, Japan and Europe. Tropical rhododendrons, generically called "Vireya" [16], grow at high altitudes in Southeast Asia (Indochina), up to the south in Indonesia, Philippines and northern parts racemes, the species has a wide range of colors, maybe one of the richest in the plant world, some parts of the flower can be poisonous. Flowering period can begin in early spring in different species until late summer, some even in fall, and the tropical ones throughout the year. The fruit is usually a woody capsule, rarely soft, with many seeds, sometimes wearing wings and appendages to facilitate air transport [16]. All species of Rhododendron form mycorrhizae with various species of fungi in a wide variety of habitats [21].
The Ericaceae family has been divided into several subfamilies, Rhododendron genus entering Rhodoreae tribe from Ericoideae subfamily, the
- subfamily
- which
- includes
- the
Ericagenus,
Bruckenthalia and Calluna, grown under similar
conditions. The genus has been subdivided into several subgenera where azaleas, formerly classified separately in Azaleagenus, are now also classified in the
123
Rhododendron genus, but with a clear status, the 5 anthers from the flower making them easily distinguishable from true rhododendrons [29]. are found in the Apuan Alps in Italy (Tuscany), which is considered a relic of the cold climate [3].
New genetic studies have shown that Ledum
2. Rhododendron myrtifolium Schott & Kotschy
species, until recently treated separately, must be placed in the Rhododendron genus, thereby a new subsection was added. Because most names from Ledum species have been used in other species of the Rhododendron genus, new names were given, for example the species Ledum palustre became
Rhododendron tomentosum and Ledum decumbens became Rhododendron subarcticum wich most often is
treated as a subspecies of Ledum palustre. Instead
Ledum groenlandicum kept the name Rhododendron groenlandicum[10,14].
- Small
- shrub
- (10-50
- cm),
- that
hascrenate,obtuse, evergreen leaves, with pink flowers and pubescent pedicels, toxic, grows especially on shady slopes and rockery [20, 5]. A Carpatho-Balkan species, oligotrophic, mesophilic, moderate to strong acidophile, calcifuge [17, 18].
It is widespread in the Carpathian Mountains
(Eastern and Southern Carpathians - Romania, western Ukraine, but missing from the Carpathians in Slovakia), in the Balkan Mountains and Rila (Bulgaria) and in northern Macedonia, Serbia, Kosovo, Albania [20, 5, 13, 27]. The species is protected in Ukraine (where populations are declining), Bulgaria and Romania [4, 18].
1. Rhododendron ferrugineum L.
Rhododendron ferrugineum is a small shrub
(30-120 cm, maximum 1.8 m) evergreen with dark green elliptical leaves, densely covered underneath with reddish-brown (rust) scales, hence the scientific name. The flowers are deep pink to purple [16, 1]. The leaves are toxic, after consumption can cause damage to the digestive, circulatory, nervous and respiratory system [29].
Material and Methods
The methods used in the study of plant communities from mountain areas are based on observation, description, collecting material for genetic and structural laboratory tests. Analysis of floristic composition and the phytocoenosis structure was made through field and laboratory activities. In order to determine the species, the following papers were used: Flora Europaea, vol. I – IV (1964 – 1980), Flora Alpina – Aeschimann 2004, Flora ilustrata a Romaniei – Ciocarlan, 2009. Research methods were based on criteria developed by Braun – Blanquetusing the surveys conducted in the summer months in both the Alps and the Carpathians (Fig.1, 2).
A Southern European species, widespread in the high mountain (subalpine) zone of the Alps
- (Southern
- Germany,
- Austria,
- Liechtenstein,
Switzerland, southeastern France, northern Italy, northwestern Slovenia), Pyrenees (southern France, northern Spain, Andorra), Jura Mountains (eastern France, north-western Switzerland and west Germany), Northern Apennines (central Italy) and the Dinaric Mountains (Slovenia, northern Croatia, Serbia, Kosovo, Macedonia, Albania) [1, 26]. It was introduced in the Czech Bohemian Massif [28]. It is protected in Austria, Italy, Slovenia, Croatia and Serbia [28].
Rhododendrons habitats researched in this paper
Habitat description was made for each species of Rhododendron depending on the geographic area it occupies. Habitats addressed in the paper are filled with official denomination of the habitats found under different names but with the same species and ecological requirements, from many scientific studies published over the years, greatly helping identification and correlation between them. The paper also presents phytocenologic surveys on plants identified in each habitat.
It forms large thickets in mountainous upper floors, in open places, steep slopes or rare forest [20].
Cold climate element, refugee in the upper floors of the mountains, rarely may occur under 1600 m. One such place is the resort Schrottkogel, southwest of Klagenfurt in Carinthia (Austria), at just 700 m altitude, it is considered a glacial relict [8]. Also it was observed that it can survive in the Sphagnum bogsin the southern Alps, Tyrol region, Italy [9] or in the Julian Alps in Slovenia together with Pinus mugo[15]. In the northern Apennines Mountains of Italy,
Rhododendron ferrugineum populations are considered relics. Rhododendron ferrugineum is found only on a
few massive such as Cima Belfiore (1810 m), Mount Prado (2053 m), Mount Libro Aperto (1937 m), where these populations grow on the northern slopes between 1750-1937 m altitude. The conservation of these populations is due to heavy rainfall and acid soil [7].
Other small populations of Rhododendron ferrugineum
Results and Discussions
Alpine and subalpine scrubland with Rhododendron
ferrugineum
Biotope: Rhododendron ferrugineum form large under
wood in rocky and grassy places, especially in the subalpine level of the Alps, along with other short or tall evergreen, alpine bushes such as Pinus mugo, resistant to severe frost, between 1600-2500 m altitudes. The
124
main spread is influenced by soil specifics, so it will be found only on acid soils with siliceous rock substrate (bedrock, granite, etc.), poor in nutrients, clayey or peaty and rich with moist humus. Plants can survive on clay soils if constant wet air masses are in the area. On the northern slopes of the French Alps was observed
that the populations of Rhododendron ferrugineum
abound especially because there is reduced grazing in the area. Rhododendrons population has declined in all Central Europe massifs where intensive grazing is practiced.
Plant associations: Rhododendretum ferruginei Rübel
1911
Structure and floristic composition: This
habitat is well represented in the Austrian Alps where
Rhododendron ferrugineum are found on podzolic acid
soils in alpine and subalpine areas often with dwarf pines and other crawling shrubs with a compact layer of moss.
Dominant species and characteristic species:
Rhododendron ferrugineum, Vaccinium myrtillus, Pinus mugo, Avenella flexuosa, Diphasiastrum alpinum, Huperzia selago, Lonicera caerulea, Pyrola minor. Other important species: Juniperus communis ssp. nana, Dryas octopetala, Polygonum bistorta, Veratrum album, Clematis alpina, Empetrum hermaphroditum, Loiseleuria procumbens, Vaccinium uliginosum ssp. pubescens.
Synonyms used for this habitat
- 4060 Alpine and Boreal heaths (EU Habitats
Directive Annex 1)
(Acidocline alpenrose heaths (Rhododendro-
Vaccinion Schnyder 1930))
- 4060 Landes subalpines acidiphiles hautes à
Rhododendron ferrugineux (CORINE)
- F2.2 Evergreen alpine and subalpine heath and scrub (EUNIS)
- 31.4 Alpine and boreal heaths (Palearctic
Habitats)
In this habitat we find many endemic plants
for the Alps like:Delphinium elatum subsp. austriacum, Pulsatilla alpina subsp. schneebergensis, Pulsatilla alpina subsp. austriaca, Cerastium pedunculatum, Cerastium charantiacum subsp. charintiacum. Other
species from the same habitat are: Vaccinium myrtillus,
Calamagrostis villosa, Valeriana celtica ssp. norica, Cystopteris montana, Vaccinium vitis-idaea, Huperzia selago, Dryopteris expansa, Solidago virgaurea ssp. minuta, Empetrum hermaphroditum, Homogyne alpina, Vaccinium gaultherioides, Oxalis acetosella.
- F2.2 Alpide acidocline [Rhododendron] heaths (EUNIS)
- 31.42 Alpide acidocline alpenrose heaths
(Palearctic Habitats)
- -
- F2.3 Subalpine and oroboreal bush
communities (EUNIS)
- -
- 31.6 Subalpine and arboreal bush
communities (Palearctic Habitats)
Fig. 1. Locations where observations were made in the Alps for Rh. Ferrugineum (red dots)
125
Fig. 2. Rhododendron ferrugineum in the Alps (Klausen Pass – 1952 m altitude - Austria)
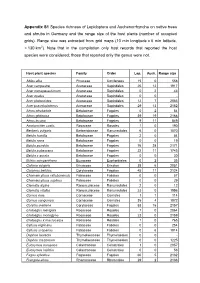
Table 1
Rhododendron ferrugineum field surveys in the Alps (Austria)
Field survey number Altitude Exposition Herb cover (%) Sample area (m2)
Rhododendron ferrugineum Vaccinium myrtillus Pinus mugo
1
1952 N
80 25
4
2
2078 NE
90 25
5
3
2101 N
70 25
4
3+
3-
2+
Larix decidua
- -
- -
- +
Gentiana burseri Vaccinium gaultherioides Juniperus sibirica Juncus trifidus
-321
+312
+212
Luzula sieberi
- +
- -
- +
Blechnum spicant Calluna vulgaris Avenella flexuosa Homogyne alpina Lonicera nigra
+-1+
++++
--2+
- -
- +
- -
Huperzia selago Melampyrum sylvaticum Pyrola minor
++-
-++
---
Nardus stricta
- +
- 1
- +
126
Gentiana punctata Anthoxanthum alpinum Geum montanum Festuca varia Orthilia secunda Arctostaphylos uva-ursi Solidago virgaurea subsp. minuta Leontodon helveticus Luzula alpinopilosa Arnica montana
-++++-+-+++-++++-
++-+--+-+--+-++++1+
--++-+++++-+--+--
Pinus cembra Agrostis rupestris Deschampsia cespitosa Empetrum nigrum subsp. hermaphroditum Poa chaixii Calamagrostis villosa Cystopteris montana Vaccinium vitis-idaea Campanula alpina
+-
+-
- Alpine and subalpine scrubland with Rhododendron
- Synonyms used for this habitat
myrtifolium
- 4060 Alpine and Boreal heaths (EU Habitats
- Directive Annex 1)
- Biotope: In Romania this habitat is frequent
on large areas in subalpine and alpine floors, especially in the juniper zone, between 1800 - 2200 (2300) m altitude with heavy rainfall (Fig. 3).
- 31.4 Alpine and Boreal heaths (CORINE)
- F2.224 Carpathian Rhododendron kotschyi
heaths (Emerald)
F2.225 Balkan Rhododendron kotschyi
heaths (Emerald)
- F2.2 Carpathian [Rhododendron kotschyi]
heaths (EUNIS)
- F2.2 Balkan [Rhododendron kotschyi] heaths
(EUNIS)
These habitats are installed on slopes and
-
ridges with northern and northeastern exposition, with medium or large inclinations. The substrate consists of siliceous rocks, rarely limestone. Soils are shallow, skeletal lithosols, sometimes on screen, with strong acid to acid reaction (pH 4.7 to 5.4). It is a habitat that requires protection, especially in Bucegi Mountains being subjected to numerous human activities with negative impact especially overgrazing, destruction of shrub vegetation to increase grassland surface in these
areas. Also Rhododendron myrtifolium is quite
sensitive to late and heavy frosts, being stronger developed only in areas with permanent snow and full coverage. The habitat has a high conservation value, sheltering many rare, vulnerable and endemic plants
such as: Campanula serrata, Salix retusa, Viola alpina, Armeria alpina.
- -
- 31.424 Carpathian Kotschy's alpenrose
heaths (Palearctic Habitats)
- 31.425 Rhodopide and Balkan Kotschy's alpenrose heaths (Palearctic Habitats)
- -
- R3104 Rhododendron scrubland southeast
(Rhododendron myrtifolium) with
Carpathian
blueberries (Vaccinium myrtillus) [6]
Plant associations Rhododendro myrtifolii –
Vaccinietum
- (Borza
- 1959)
- Boscaiu
- 1971.
127
Fig. 3. Locations where observations were made in the Carpathian Mountains for Rh. myrtifolium
scoparium, Hylocomyum splendens, Polytrichum juniperinum. Other important species: Bruckenthalia spiculifolia, Pinus mugo, Juniperus sibirica, Campanula abietina, Campanula serrata, Pinus cembra, Carex atrata, Avenula versicolor, Picea abies, Luzula sylvatica, Soldanella hungarica ssp.major, Calamagrostis villosa, Lonicera caerulea, Oxalis acetosella, Deschampsia flexuosa, Melampyrum sylvaticum, Huperzia selago, Lycopodium annotinum, Potentilla aurea ssp. chrysocraspeda.Rhododendron
myrtifolium under wood is widespread in Bucegi Mountains where they have an ecological role in
Structure and floristic composition:
Generally the species involved are of alpine, circumpolar and boreal origin, most of them acidophilus. Phytocoenosis is primary, but may extend secondary on degraded soils, deforested spruce and juniper forest trees. Dominant species and
characteristic: Rhododendron myrtifolium (Rh. kotschy) (Fig. 4), Vaccinium myrtillus, Vaccinium vitis- idaea,Vaccinium gaultherioides, Saxifraga paniculata, Campanula kladniana. They achieve coverage of 80-
100%. Grasses layer is dominated by: Nardus stricta,
Anthoxanthum odoratum, Luzula luzuloides, Potentilla ternata, Homogyne alpina, Loiseleuria procumbens, Geum montanum and a series of moss: Dicranum
- stabilizing
- coast
- and
- screens.
Fig. 4. Rhododendron myrtifolium in Bucegi Mountains (Furnica Mountain – 2028 m altitude)
128
Table 2
Rhododendron myrtifoliumsurvey in the Carpathian Mountains (Retezat)
Survey number Altitude Exposition Herb cover (%)
1
1990 NE
90 25
4321+-
2
2025 N
70 25
532+++++-+-++-+---+-------
3
2040 N
60 25
4211+++++----+-----+-++-+-+---+-+-
Sample area (m2)
Rhododendron myrtifolium Vaccinium gaultherioides Vaccinium myrtillus Vaccinium vitis-idaea Campanula alpina Saxifraga paniculata Campanula kladniana Nardus stricta Anthoxanthum odoratum Potentilla ternata Bruckenthalia spiculifolia Primula minima Homogyne alpina Festuca supina Luzula luzuloides Loiseleuria procumbens Geum montanum Pinus cembra Campanula serrata Luzula sylvatica
11++++++++++-+++-+-
Calamagrostis villosa Avenula versicolor Lonicera caerulea Melampyrum sylvaticum Carex atrata Soldanella hungarica subsp. major Deschampsia flexuosa Huperzia selago
+
- -
- +
+---
++++++-+-+-
Picea abies Lycopodium annotinum Potentilla aurea subsp. chrysocraspeda Dianthus gelidus Juncus trifidus Oxyria dygina Thymus pulcherrimus Viola alpina Senecio carpaticus Oxytropis halleri
-+++--+
++-+
From observations made on the field can conclude that these habitats have a fragile structure and can be easily affected by human activities such as excessive grazing and tourist activities.
Conclusions
- Rhododendron
- ferrugineum