Of Polymyxin B Solutions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(From the Department of Physiology, University of Minnesota, Minneapolis) Same Journal. N. K., Kolloid-Z., 1929, 47, 101. the Jo
THE STRUCTURE OF THE COLLODION MEMBRANE AND ITS ELECTRICAL BEHAVIOR II, THE ACTIVATED COLLODION MEMBRANE BY KARL SOLLNER, IRVING ABRAMS, AND CHARLES W. CARR (From the Department of Physiology, University of Minnesota, Minneapolis) (Received for pubfication, March 31, 1941) I In preceding communicationst, 2 we were led to the conclusion that the elec- trochemical activity of collodion membranes, as manifested by concentration potentials, etc., is due principally to acidic impurities. Accordingly, different brands of collodion differ widely as to their activity, the purer brands being less active. The impure (but active) foreign brands of collodion, heretofore generally used by workers in the field of electrochemical membrane investiga- tion, are no longer obtainable. In order to continue our investigation, it became necessary to find methods to produce active collodion membranes at will. The idea of inducing changes in the electrochemical characteristics of mem- branes is not entirely new. Many investigators have activated membranes by the adsorption of proteins, e.g. the proteinized membranes of Loeb. 8 Other investigators use other organic compounds, usually dyestuffs. These may be adsorbed like proteins or they may be dissolved in the collodion solution* pre- vious to casting the membranes. Such membranes are interesting and useful in their own right, but are not altogether satisfactory substitutes for active collodion membranes. They very often show considerable asymmetry; more- over, the dyestuffs so far employed (according to the literature) are slowly re- leased into the solution in contact with the membrane, whereby the character of the membrane is considerably changed. Meyer and Sievers5 used an oxida- tion method to activate a cellophane membrane. -

NACE Bromine Chemistry Review Paper
25 YEARS OF BROMINE CHEMISTRY IN INDUSTRIAL WATER SYSTEMS: A REVIEW Christopher J. Nalepa Albemarle Corporation P.O. Box 14799 Baton Rouge, LA 70898 ABSTRACT Bromine chemistry is used to great advantage in nature for fouling control by a number of sessile marine organisms such as sponges, seaweeds, and bryozoans. Such organisms produce small quantities of brominated organic compounds that effectively help keep their surfaces clean of problem bacteria, fungi, and algae. For over two decades, bromine chemistry has been used to similar advantage in the treatment of industrial water systems. The past several years in particular has seen the development of several diverse bromine product forms – one-drum stabilized bromine liquids, all-bromine hydantoin solids, and pumpable gels. The purpose of this paper is to review the development of bromine chemistry in industrial water treatment, discuss characteristics of the new product forms, and speculate on future developments. Keywords: Oxidizing biocide, bleach, bromine, bromine chemistry, sodium hypobromite, activated sodium bromide, Bromochlorodimethylhydantoin, Bromochloromethyethylhydantoin, Dibromodi- methylhydantoin,, BCDMH, BCMEH, DBDMH, stabilized bromine chloride, stabilized hypobromite INTRODUCTION Sessile marine organisms generate metabolites to ward off predators and deter attachment of potential micro- and macrofoulants. Sponges, algae, and bryozoans for example, produce a rich variety of bromine-containing compounds that exhibit antifoulant properties (Fig. 1).1,2,3 Scientists are actively studying these organisms to understand how they maintain surfaces that are relatively clean and slime- free.4 Brominated furanones isolated from the red algae Delisea pulchra, for example, have been found to interfere with the chemical signals (acylated homoserine lactones) that bacteria use to communicate with one another to produce biofilms.5,6 This work may eventually lead to more effective control of microorganisms in a number of industries such as industrial water treatment, oil and gas production, health care, etc. -

(12) United States Patent (10) Patent No.: US 6,270,722 B1
USOO6270722B1 (12) United States Patent (10) Patent No.: US 6,270,722 B1 Yang et all e 45) Date of Patent:e Aug. 7, 2001 (54) STABILIZED BROMINE SOLUTIONS, (56) References Cited METHOD OF MANUFACTURE AND USES THEREOF FOR BOFOULING CONTROL U.S. PATENT DOCUMENTS 3,328.294 6/1967 Self et al.. (75) Inventors: Shunong Yang; William F. McCoy, 3,558,503 1/1971 Goodenough et al.. both of Naperville; Eric J. Allain, 3,767,586 10/1973 Rutkiewic et al.. Aurora; Eric R. Myers, Aurora; 5,683,654 11/1997 Dalmier et al.. Anthony W. Dalmier, Aurora, all of IL 5,795,487 8/1998 Dalmier et al.. (US) 6,007,726 * 12/1999 Yang et al. ........................ 422/37 X 6,015,782 1/2000 Petri et al. ........................... 510/379 (73) Assignee: Nalco Chemical Company, Naperville, 6,110,387 * 8/2000 Choudhury et al. ................. 210/752 IL (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this WO 97/20909 6/1997 (WO). patent is extended or adjusted under 35 WO 97/43392 11/1997 (WO). U.S.C. 154(b) by 0 days. * cited by examiner Primary Examiner Elizabeth McKane (21) Appl. No.: 09/283,122 (74) Attorney, Agent, or Firm-Kelly L. Cummings; (22) Filed: Mar. 31, 1999 Thomas M. Breininger (51) Int. Cl." ................................ A61L 2/16; C01B 709; (57) ABSTRACT D06L 3/06 Stabilized bromine Solutions are prepared by combining a (52) U.S. Cl. ..................................... 422/37; 422/6; 8/107; bromine Source and a Stabilizer to form a mixture, adding an 8/137; 162/1; 423/500; 252/187.2 oxidizer to the mixture, and then adding, an alkaline Source (58) Field of Search ................................... -

Commission Delegated Regulation (Eu
8.2.2019 EN Official Journal of the European Union L 37/1 II (Non-legislative acts) REGULATIONS COMMISSION DELEGATED REGULATION (EU) 2019/227 of 28 November 2018 amending Delegated Regulation (EU) No 1062/2014 as regards certain active substances/product- type combinations for which the competent authority of the United Kingdom has been designated as the evaluating competent authority (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products (1) and in particular the first subparagraph of Article 89(1) thereof, Whereas: (1) Commission Delegated Regulation (EU) No 1062/2014 (2) sets out in its Annex II a list of active sub stance/product-type combinations included in the programme of review of existing active substances contained in biocidal products (‘review programme’). (2) The competent authority of the United Kingdom of Great Britain and Northern Ireland (‘the United Kingdom’) is the evaluating competent authority for several active substance/product-type combinations listed in Annex II to Delegated Regulation (EU) No 1062/2014. (3) The United Kingdom submitted on 29 March 2017 the notification of its intention to withdraw from the Union pursuant to Article 50 of the Treaty on European Union. As a result, the United Kingdom will withdraw from the Union on 30 March 2019 and the Union legislation will no longer apply to and in the United Kingdom. -

United States Patent (19) 11 Patent Number: 5,011,997 Hazen Et Al
United States Patent (19) 11 Patent Number: 5,011,997 Hazen et al. (45) Date of Patent: Apr. 30, 1991 54 PROCESS FOR 2,822,396 2/1958 Dent ............................... 564/414 X BIS(4-AMINOPHENYL)HEXAFLUOROPRO. 3,897,498 7/1975 Zengel et al. ....................... 564/44 PANE 3,904,69 9/1975 Carnmam et al. ............. 564/414 X 4,082,749 4/1978 Quadbeck-Seeger et al. ..... 564/44 (75) Inventors: James R. Hazen, Coventry; William X R. Lee, E. Providence, both of R.I. 4,198,348 4/1980 Bertini et al. ....................... 564/44 (73) Assignee: Hoechst Celanese Corp., Somerville, OTHER PUBLICATIONS N.J. Houben-Weyl, "Stickstoff Verbindungen', Methoden 21 Appl. No.: 105,857 der Organischen Chemie, vol. 10, Part 3, pp. 375–377 (1965). 22 Fied: Oct. 7, 1987 Primary Examiner-Glennon H. Hollrah 51 Int. C. ................... CO7C 211/50; CO7C 209/50 Attorney, Agent, or Firm-Perman & Green (52) U.S.C. .................................... 564/335; 564/330; 564/414 57 ABSTRACT 58 Field of Search ............... 564/330, 158, 335, 181, This invention is an improved process for preparing 564/.414 2,2-bis(4-aminophenyl)hexafluoropropane which com prises subjecting 2,2-bis(4-aminocarbonylphenyl)hexa 56) References Cited fluoropropane to the Hofmann reaction and then treat U.S. PATENT DOCUMENTS ing that reaction product under reducing conditions to 902,150 0/1908 Heidenreich ................... 564/414 X produce the desired amine in high yield. 1,850,526 3/1932 Zitscher .......................... 564/.414 X 2,334,201 11/1943 Kamm et al. ................... 564/414 X 7 Claims, No Drawings 5,011,997 1. -

1. Give the Correct Names for Each of the Compounds Listed Below. A
1. Give the correct names for each of the compounds listed below. a) NaCl sodium chloride n) ZrS2 zirconium sulfide b) FrBr francium bromide o) AgI silver iodide c) KF potassium fluoride p) BaSe barium selenide d) RaS radium sulfide q) MgO magnesium oxide e) LiI lithium iodide r) LaBr3 lanthanum bromide f) Li3N lithium nitride s) Sr3N2 strontium nitride g) AlBr3 aluminum bromide t) Cd3As2 cadmium arsenide h) CdCl2 cadmium chloride u) Rb2Se rubidium selenide i) K2O potassium oxide v) Rb3N rubidium nitride j) InF3 indium fluoride w) BaF2 barium fluoride k) ZnO zinc oxide x) ZrTe2 zirconium telluride l) Y2O3 yttrium oxide y) Cs3P cesium phosphide m) CaTe calcium telluride z) Y2O3 yttrium oxide 2. Write the correct chemical formula for each of the following compounds. a) potassium bromide KBr n) potassium nitride K3N b) zinc bromide ZnBr2 o) aluminum bromide AlBr3 c) lithium iodide LiI p) zinc phosphide Zn3P2 d) scandium chloride ScCl3 q) magnesium sulfide MgS e) magnesium chloride MgCl2 r) hafnium chloride HfCl4 f) magnesium oxide MgO s) barium sulfide BaS g) hydrogen sulfide H2S t) tantalum oxide Ta2O5 h) gallium iodide GaI3 u) zirconium nitride Zr3N4 i) sodium oxide Na2O v) potassium selenide K2Se j) magnesium selenide MgSe w) germanium fluoride GeF4 k) calcium fluoride CaF2 x) francium phosphide Fr3P l) aluminum oxide Al2O3 y) zinc arsenide Zn3As2 m) beryllium chloride BeCl2 z) scandium telluride Sc2Te3 L. h. s. – Chemistry – Nomenclature – Answers – Page 1 3. Give the correct names for each of the compounds listed below. a) CaSO4 calcium -

PRIZE LIST 2010 Edited by Viral
PRICE LIST 2013 NILE CHEMICALS pH INDICATORS , BIOLOGICAL STAINS CATALYST FOOD COLOURS CHEMICALS (LAB & BULK) REAGENTS (LAB & BULK) LABO ATORY REAGENTS & FINE CHEMICALS PRODUCT PROFILE PURE, EXTRA PURE, PURIFIED We have pure , extra pure and purified products , available as per buyers requirements and specifications. GUARANTEED REAGENTS (GR) Our Guaranteed Reagents are used for analytical and research work, where high purity is essential. This is similar to grade marketed by other International company. HPLC GRADE This are high performance chemicals , used in liquid chromatography used frequently in biochemistry and analytical chemistry to separate, identify, and quantify compounds SPECTROSCOPY GRADE These are solvents of high optical purity for UV/Visible/IR/Fluorescence/NMR and Mass spectroscopy. BIOLOGICAL STAIN Our Ph Indicators are ready to use solutions for Microbiology, Histology, Hematology and Cytology and are at par with certified grade stains as per International norms. Address : 202,Matruchhay ,378 /80 Narshi Natha Street ,Masjid , Mumbai -400 009 INDIA Email : [email protected] Tel: 91 22 66313162 : Fax : 91 22 23454828 Web site www.nilechemicals.com NILE CHEMICALS EXPLOSIVES: Certain Substances reacts exothermically, generating gases, which may explode on heating, hence storage may be required in water or suitable solvent OXIDISING:Some Substance reacts with oxygen and may cause fire hazard TOXIC:Certain substance on contact with human body may cause acute or chronic damage HARMFUL:Certain substance on contact with human body may cause acute or chronic damage FLAMABLE:Chemicals , Solvents with a flash point below zero degree and boiling point of 35 deg c are termed as flammable items CORROSIVE:Substance which causes severe damage to living tissues IRRITANT:Substance which causes irritation on contact with skin etc DANGEROUS: substance which causes damages to ecosystem RADIOACTIVE:Substance which evolves radiation in the ecosystem NILE CHEMICALS PRICE LSIT 2013 CAT.NOS. -

Sodium Chlorite Handbook
SODIUM CHLORITE HANDBOOK OxyChem Technical Information 01/2015 Dallas-based Occidental Chemical Corporation is a leading North American manufacturer of basic chemicals, vinyls and performance chemicals directly and through various affiliates (collectively, OxyChem). OxyChem is also North America's largest producer of sodium chlorite. As a Responsible Care® company, OxyChem's global commitment to safety and the environment goes well beyond compliance. OxyChem's Health, Environment and Safety philosophy is a positive motivational force for our employees, and helps create a strong culture for protecting human health and the environment. Our risk management programs and methods have been, and continue to be, recognized as some of the industry's best. OxyChem offers an effective combination of industry expertise, experience, on line business tools, quality products and exceptional customer service. As a member of the Occidental Petroleum Corporation family, OxyChem represents a rich history of experience, top-notch business acumen, and sound, ethical business practices. HB-600 2 01/2015 Table of Contents Introduction to Sodium chlorite ................................................................................................................. 4 Manufacturing ................................................................................................................................................ 4 Sodium Chlorite End Uses ............................................................................................................................ -

(12) United States Patent (10) Patent No.: US 8,293,918 B2 Modi Et Al
US008293918B2 (12) United States Patent (10) Patent No.: US 8,293,918 B2 Modi et al. (45) Date of Patent: Oct. 23, 2012 (54) PROCESS FOR PRODUCING (52) U.S. Cl. ....................................................... 54.6/345 DHALOPYRIDINES (58) Field of Classification Search ........................ None See application file for complete search history. (75) Inventors: Gelebith H. Modi, Noida (IN); Anil Kumar Tyagi, Noida (IN); Ashutosh Agarwal, Noida (IN); Hem Chandra, (56) References Cited Gajraula (IN); Nikhilesh Chandra Bhardwaj, Gajraula (IN); Pradeep FOREIGN PATENT DOCUMENTS Kumar Verma, Gajraula (IN) WO WO 2005070888 A2 * 8, 2005 (73) Assignee: Jubilant Organosys Limited, Noida OTHER PUBLICATIONS (IN) Kovacic J Amer Chem Soc 1954 vol. 76 pp. 5491-5494.* Streitwieser, A. et al., Introduction to Organic Chemistry, 4th ed., (*) Notice: Subject to any disclaimer, the term of this 1992, p. 793.* patent is extended or adjusted under 35 Sigma-Aldrich Catalogue, 2009-2010, p. 2717.* U.S.C. 154(b) by 315 days. * cited by examiner (21) Appl. No.: 12/641,926 Primary Examiner — Janet Andres (22) Filed: Dec. 18, 2009 Assistant Examiner — Heidi Reese (74) Attorney, Agent, or Firm — Oblon, Spivak, (65) Prior Publication Data McClelland, Maier & Neustadt, L.L.P. US 2010/0160641 A1 Jun. 24, 2010 (57) ABSTRACT (30) Foreign Application Priority Data Disclosed herein an improved process for producing 2.3- (IN) ........................... 2891 (DELA2008 dihalopyridine with high purity at industrial scale with mini Dec. 19, 2008 mum effluent. (51) Int. C. C07D 21 1/72 (2006.01) 23 Claims, No Drawings US 8,293,918 B2 1. 2 PROCESS FOR PRODUCING 3-aminopyridine with oxydol at a molar ratio of 1:1 in con DHALOPYRIDINES centrated hydrochloric acid at 6-8°C. -

New Reactions of Nitro Compounds
This dissertation has been 62-2176 microfilmed exactly as received KAPLAN, Ralph Benjamin, 1920- NEW REACTIONS OF NITRO COMPOUNDS. The Ohio State University, Ph.D., 1950 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan NEW REACTIONS OF NITRO COMPOUNDS DISSERTATION Presented In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By RALPH B. KAPLAN, B.A., The Ohio State University 1950 Approved by: Adviser TABLE OF CONTENTS Page INTRODUCTION Acknowledgments Statement of the Problem SECTION I REACTION OF SODIUM NITROALKANES WITH VARIOUS NITRATING AGENTS 1 I. Discussion 1 A. Introduction 1 B. Agents Investigated 2 1. Nltryl Chloride 2 a. Preparation 2 b. Review of thé Reactions of Nltryl Chloride With Organic Reagents U c. Reaction of Sodium 2-Nltropropane With Nltryl Chloride 6 d. Reaction of Sodium 2-Nltropropane with Nltryl Chloride and Aluminum Chloride 11 2. Mixed Nitric and Sulfuric Acid 12 a. Reaction of Sodium 2-Nltropropane With Mixed Nitric and Sulfuric Acid 12 3. Methyl Nitrate Ih a. Reaction of Sodium 2-Nltropropane and of Potassium Nltroethane l4 I. Alkyl Nitrates as Nitrating Agents 14 II. Discussion of Results 15 Page II. Experimental 21 A. Agents Investigated 21 1. Nltryl Chloride 21 a. Preparation 21 1. Intermediates; Chlorosulfonlc Acid and Nitric Acid, Anhydrous 11. Procedure for Making Nltryl-Chloride h. Reaction of Nltryl Chloride with a Secondary Nltroalkane 2k 1. 2-Nltropropane 11. Sodium 2-Nltropropane c. Reaction of Sodium 2-Nltropropane, Nltryl Chloride and Aluminum Chloride. 27 2. Reaction of Sodium 2-Nltropropane With Mixed Nitric and Sulfuric Acids 28 3. -
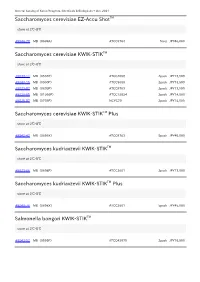
Saccharomyces Cerevisiae EZ-Accu Shottm
General Catalog of Kanto Reagents, Chemicals & Biologicals + Oct. 2021 Saccharomyces cerevisiae EZ-Accu ShotTM store at 2℃-8℃ 49944-78 MB(0699A) ATCC9763 5test JPY46,000 Saccharomyces cerevisiae KWIK-STIKTM store at 2℃-8℃ 49843-14 MB(0534P) ATCC4098 2pack JPY13,500 49843-15 MB(0900P) ATCC9080 2pack JPY13,500 49823-87 MB(0699P) ATCC9763 2pack JPY13,500 49820-65 MB(01066P) ATCC18824 2pack JPY14,500 49825-87 MB(0736P) NCYC79 2pack JPY14,500 Saccharomyces cerevisiae KWIK-STIKTM Plus store at 2℃-8℃ 49842-47 MB(0699X) ATCC9763 5pack JPY45,000 Saccharomyces kudriavzevii KWIK-STIKTM store at 2℃-8℃ 49823-86 MB(0698P) ATCC2601 2pack JPY13,500 Saccharomyces kudriavzevii KWIK-STIKTM Plus store at 2℃-8℃ 49840-76 MB(0698X) ATCC2601 5pack JPY45,000 Salmonella bongori KWIK-STIKTM store at 2℃-8℃ 49843-52 MB(0595P) ATCC43975 2pack JPY16,500 General Catalog of Kanto Reagents, Chemicals & Biologicals + Oct. 2021 Salmonella enterica subsp.arizonae EZ-Accu ShotTM store at 2℃-8℃ 49946-13 MB(0901A) ATCC13314 5test JPY46,000 Salmonella enterica subsp.arizonae KWIK-STIKTM store at 2℃-8℃ 49823-91 MB(0901P) ATCC13314 2pack JPY13,500 Salmonella enterica subsp.diarizonae KWIK-STIKTM store at 2℃-8℃ 49820-33 MB(01054P) ATCC12325 2pack JPY14,500 49844-90 MB(01045P) ATCC29934 2pack JPY14,500 Salmonella enterica subsp.enterica serotype Abony EZ-CFUTM store at 2℃-8℃ 49840-79 MB(0890C) NCTC6017 10test JPY87,000 Salmonella enterica subsp.enterica serotype Abony EZ-CFUTM One Step store at 2℃-8℃ 49842-70 MB(0890Z) NCTC6017 10test JPY86,000 Salmonella enterica subsp.enterica serotype Abony KWIK-STIKTM store at 2℃-8℃ 49825-88 MB(0890P) NCTC6017 2pack JPY11,500 General Catalog of Kanto Reagents, Chemicals & Biologicals + Oct. -

A Colorimetric Method for the Determination of the Principal Metabolites of Nicotinic Acid in Human Urine
Vol. 56 ORIENTATION OF GROUPS IN PROTEIN MONOLAYERS 513 Lysyl Bulky 2. At pH 13 the un-ionized c-NH, groups ofthe residue hydrocarbon lysine residues tend to enter the non-aqueous phase, oil oil air roup Peptide \ group especially at the air-water interface when the chain z z Water neighbouring amino acid residues in the peptide EvQ_OI O chain are favourable. 3. The 'unavailability' ofsuch e-NH2 groups at water water ork Glutamyl this interface and in the native protein residue may be of O/W:. low and O/W: very high A/W: moderate major biological importance. mnoderate film pressures film pressures film pressures The author is deeply indebted to Prof. Sir Eric Rideal, (1-6 m.'I mg:-') (< 1-0 M., mg.-) (1-6 m.1 mg.-') F.R.S., for his continued encouragement throughout the (a) (b) course of this work. Fig. 4. Orientations of the -(CH2)4NH2 chains in proteins at (a) the oil-water interface, and (b) the air-water 1,EFERENCES interface. Alexander, A. E. & Teorell, T. (1939). Tranm. Faraday Soc. in 35, 727. the aqueous phase either A/W monolayers at Cohn, E. J. & Edsall, J. T. (1943). Protein8, Amino-acids and moderate pressures or in the native protein (where Peptide8, pp. 358-60. New York: Reinhold. the van der Waals forces of cohesion between the Davies, J. T. (1951 a). Nature, Lond., 167, 193; Z. Elektro- hydrocarbon side chains are also very powerful). chem. 55, 559. When the protein is unfolded at a lipid-water Davies, J. T. (1951 b).